Abstract
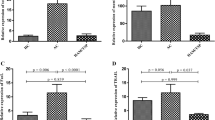
Interleukin-21 (IL-21) enhances the survival and cytotoxic properties of cytotoxic T cells (CTLs) and exhibits essential roles in controlling chronic viral infections. HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a chronic progressive inflammatory disease of the nervous system. The main determinant of disease progression is efficiency of the CTL response to Human T lymphotropic virus types I (HTLV-1). In this study, the expression of host IL-21 and HTLV-I Tax and proviral load (PVL) was evaluated to understand the role and mechanism of IL-21 in HTLV-1 infections and the subsequent development of HAM/TSP. A cross-sectional study was carried out on 20 HAM/TSP patients, 20 asymptomatic HTLV-1 carriers (ACs) and 20 healthy controls (HCs) to evaluate the expression of IL-21 and Tax and PVL in non-activated and phorbol myristate acetate (PMA)-ionomycin-activated peripheral blood mononuclear cells (PBMCs). The mean mRNA expression of IL-21 in the non-activated and activated PBMCs was higher (by 5–13 times) in the HAM/TSP patients than in ACs and HCs (p < 0.05); however, there was no significant difference between ACs and HCs. In contrast to the IL-21 mRNA expression, the serum level of the IL-21 protein was significantly lower in the HAM/TSP patients than in ACs and HCs (p < 0.05). Furthermore, higher expression of Tax and PVL was observed in the HAM/TSP subjects than ACs (p < 0.05). In addition, Tax gene expression was positively correlated with PVL (R = 0.595, p = 0.000) and IL-21 gene expression (R = 0.395, p = 0.021) in the HTLV-1-infected subjects. In conclusion, the increase in IL-21 mRNA expression may reflect the attempt of infected T cells to induce an appropriate antiviral response, and the decrease in IL-21 protein expression may reflect the inhibition of IL-21 mRNA translation by viral factors in favour of virus evasion and dissemination.




Similar content being viewed by others
References
Bangham CRM, Araujo A, Yamano Y, Taylor GP (2015) HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat Rev Dis Prim 1:15012. doi:10.1038/nrdp.2015.12
Rafatpanah H, Hedayati-Moghaddam MR, Fathimoghadam F et al (2011) High prevalence of HTLV-I infection in Mashhad, Northeast Iran: a population-based seroepidemiology survey. J Clin Virol 52:172–176. doi:10.1016/j.jcv.2011.07.004
Boostani R, Vakili R, Hosseiny SS et al (2015) Triple therapy with prednisolone, pegylated interferon and sodium valproate improves clinical outcome and reduces human T-cell leukemia virus type 1 (HTLV-1) proviral load, tax and HBZ mRNA expression in patients with HTLV-1-associated myelopathy/tropical. Neurother 12:887–895. doi:10.1007/s13311-015-0369-3
Yari A, Rezaee SA, Valizadeh N et al (2014) Evaluation of HTLV-1 activity in HAM/TSP patients using proviral load and Tax mRNA expression after In Vitro lymphocyte activation. Iran J BasicMed Sci 17:531–536
Bangham CR (2008) HTLV-1 infection: role of CTL efficiency. Blood 112:2176–2177. doi:10.1182/blood-2008-06-163071
Toulza F, Heaps A, Tanaka Y et al (2008) High frequency of CD4 + FoxP3 + cells in HTLV-1 infection: inverse correlation with HTLV-1-specific CTL response. Blood 111:5047–5053. doi:10.1182/blood-2007-10-118539
Massoud R, Enose-akahata Y, Tagaya Y, et al (2015) Common γ-chain blocking peptide reduces in vitro immune activation markers in HTLV-1-associated myelopathy/tropical spastic paraparesis. 112:2–7. doi:10.1073/pnas.1412626112
Levin MC, Lee SM, Kalume F, et al (2009) Neurological disease. 8:509–513. doi:10.1038/nm0502-509. Autoimmunity
Tamayo O (2005) Autoimmunity and molecular mimicry in tropical spastic paraparesis/human myelopathy. 38:241–250
Iannello A, Boulassel MR, Samarani S et al (2010) Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J Immunol 184:114–126. doi:10.4049/jimmunol.0901967
Brandt K, Singh PB, Bulfone-Paus S, Ruckert R (2007) Interleukin-21: a new modulator of immunity, infection, and cancer. Cytokine Growth Factor Rev 18:223–232. doi:10.1016/j.cytogfr.2007.04.003
Spolski R, Leonard WJ (2008) Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol 26:57–79. doi:10.1146/annurev.immunol.26.021607.090316
Publicover J, Goodsell A, Nishimura S et al (2011) IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest 121:1154–1162. doi:10.1172/JCI44198
Yi JS, Du M, Zajac AJ (2009) A vital role for interleukin-21 in the control of a chronic viral infection. Science 324:1572–1576. doi:10.1126/science.1175194
Jones RB, Ndhlovu LC, Barbour JD et al (2008) Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 205:2763–2779. doi:10.1084/jem.20081398
Day CL, Kaufmann DE, Kiepiela P et al (2006) PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354. doi:10.1038/nature05115
Petrovas C, Casazza JP, Brenchley JM et al (2006) PD-1 is a regulator of virus-specific CD8 + T cell survival in HIV infection. J Exp Med 203:2281–2292. doi:10.1084/jem.20061496
Trautmann L, Janbazian L, Chomont N et al (2006) Upregulation of PD-1 expression on HIV-specific CD8 + T cells leads to reversible immune dysfunction. Nat Med 12:1198–1202. doi:10.1038/nm1482
Blackburn SD, Shin H, Haining WN et al (2009) Coregulation of CD8 + T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10:29–37. doi:10.1038/ni.1679
Frohlich A, Kisielow J, Schmitz I, et al (2009) IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324:1576–1580. doi:10.1126/science.1172815
Elsaesser H, Sauer K, Brooks DG (2009) IL-21 is required to control chronic viral infection. Science 324:1569–1572. doi:10.1126/science.1174182
Chevalier MF, Julg B, Pyo A et al (2011) HIV-1-specific interleukin-21 + CD4 + T cell responses contribute to durable viral control through the modulation of HIV-specific CD8 + T cell function. J Virol 85:733–741. doi:10.1128/JVI.02030-10
Yue FY, Lo C, Sakhdari A et al (2010) HIV-specific IL-21 producing CD4 + T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J Immunol 185:498–506. doi:10.4049/jimmunol.0903915
Iannello A, Boulassel MR, Samarani S et al (2010) IL-21 enhances NK cell functions and survival in healthy and HIV-infected patients with minimal stimulation of viral replication. J Leukoc Biol 87:857–867. doi:10.1189/jlb.1009701
Peluso I, Fantini MC, Fina D et al (2007) IL-21 Counteracts the Regulatory T Cell-Mediated Suppression of Human CD4+ T Lymphocytes. J Immunol 178:732–739. doi:10.4049/jimmunol.178.2.732
Mizuguchi M, Asao H, Hara T et al (2009) Transcriptional activation of the interleukin-21 gene and its receptor gene by human T-cell leukemia virus type 1 Tax in human T-cells. J Biol Chem 284:25501–25511. doi:10.1074/jbc.M109.010959
Ueda M, Imada K, Imura A et al (2005) Expression of functional interleukin-21 receptor on adult T-cell leukaemia cells. Br J Haematol 128:169–176. doi:10.1111/j.1365-2141.2004.05255.x
Rafatpanah H, Rezaee A, Etemadi MM et al (2012) The impact of interferon-alpha treatment on clinical and immunovirological aspects of HTLV-1-associated myelopathy in northeast of Iran. J Neuroimmunol 250:87–93. doi:10.1016/j.jneuroim.2012.05.004
Saito M, Tanaka R, Arishima S et al (2013) Increased expression of OX40 is associated with progressive disease in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. Retrovirology 10:51. doi:10.1186/1742-4690-10-51
Abdelbary NH, Abdullah HM, Matsuzaki T et al (2011) Reduced Tim-3 expression on human T-lymphotropic virus type I (HTLV-I) Tax-specific cytotoxic T lymphocytes in HTLV-I infection. J Infect Dis 203:948–959. doi:10.1093/infdis/jiq153
Everly DN Jr, Feng P, Mian IS, Read GS (2002) mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: genetic and biochemical evidence that Vhs is a nuclease. J Virol 76:8560–8571
Matis J, Kudelova M (2001) Early shutoff of host protein synthesis in cells infected with herpes simplex viruses. Acta Virol 45:269–277
Matsuura E, Kubota R, Tanaka Y (2015) Visualization of HTLV-1 Y specific cytotoxic T lymphocytes in the spinal cords of patients with HTLV-1 Y associated myelopathy/tropical spastic paraparesis. 74:2–14
Marcondes MC, Burudi EM, Huitron-Resendiz S et al (2001) Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol 167:5429–5438
Liu SM, Lee DH, Sullivan JM et al (2011) Differential IL-21 signaling in APCs leads to disparate Th17 differentiation in diabetes-susceptible NOD and diabetes-resistant NOD. Idd3 mice. J Clin Invest 121:4303–4310. doi:10.1172/JCI46187
Liu SM, King C (2013) IL-21-producing Th cells in immunity and autoimmunity. J Immunol 191:3501–3506. doi:10.4049/jimmunol.1301454
Bubier JA, Sproule TJ, Foreman O et al (2009) A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci U S A 106:1518–1523. doi:10.1073/pnas.0807309106
Gallegos AM, Bevan MJ (2004) Driven to autoimmunity: the nod mouse. Cell 117:149–151
Monteleone G, Monteleone I, Fina D et al (2005) Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn’s disease. Gastroenterology 128:687–694
Fina D, Sarra M, Fantini MC et al (2008) Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology 134:1038–1048. doi:10.1053/j.gastro.2008.01.041
Spolski R, Kashyap M, Robinson C et al (2008) IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc Natl Acad Sci U S A 105:14028–14033. doi:10.1073/pnas.0804358105
Herber D, Brown TP, Liang S et al (2007) IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol 178:3822–3830
Young DA, Hegen M, Ma HL et al (2007) Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum 56:1152–1163. doi:10.1002/art.22452
Acknowledgements
The authors express their appreciation to Dr. Mahmoud Reza Azarpazhooh in the Department of Neurology, Mashhad University of Medical Sciences and Dr. Reza Faridhosseini, Dr. Bahareh Fazeli, Mr. Mehdi Felegari, Mr. Farzad MollaHosseini, Mrs. Atefe Yari for their kind assistance. This study was financially supported by Vice Chancellor for Research, Mashhad University of Medical Sciences, Mashhad, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee. The study was approved by ethics committee of Mashhad University of Medical sciences, Mashhad (No: 1961).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
There is no conflict of interest for this study.
Rights and permissions
About this article
Cite this article
Rajaei, T., Farajifard, H., Rafatpanah, H. et al. Role of IL-21 in HTLV-1 infections with emphasis on HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Med Microbiol Immunol 206, 195–201 (2017). https://doi.org/10.1007/s00430-017-0492-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-017-0492-3