Abstract
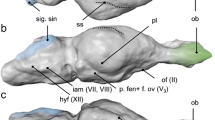
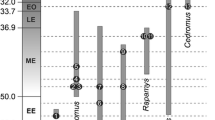
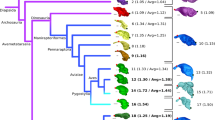
Caviomorph rodents are an exceptional model for studying the effects of ecological factors and size relations on brain evolution. These mammals are not only speciose and ecologically diverse but also present wide body size disparity, especially when considering their fossil relatives. Here, we described the brain anatomy of the largest known rodent, Josephoartigasia monesi, uncovering distinctive features within this species regarding other taxa. Albeit resembling extant pacarana Dinomys branickii, J. monesi stands out due to its longer olfactory tract and well-developed sagittal sinus. Challenging the previous hypothesis that giant rodents possessed comparatively smaller brains, we found that J. monesi and another giant extinct rodent, Neoepiblema acreensis, are within the encephalization range of extant caviomorphs. This was unraveled while developing the a Phylogenetic Encephalization Quotient (PEQ) for Caviomorpha. With PEQ, we were able to trace brain-size predictions more accurately, accounting for species-shared ancestry while adding the extinct taxa phenotypic diversity into the prediction model. According to our results, caviomorphs encephalization patterns are not the product of ecological adaptations, and brain allometry is highly conservative within the clade. We challenge future studies to investigate caviomorphs encephalization within different taxonomic ranks while increasing the sampled taxa diversity, especially of extinct forms, in order to fully comprehend the magnitude of this evolutionary stasis.







Similar content being viewed by others
Data availability
The article’s supporting data and R scripts can be found in the Supporting Material and at the git-hub repository (https://github.com/jbubadue/Ferreira_et_al_Jmonesi).
References
Álvarez A, Arévalo RLM, Verzi DH (2017) Diversification patterns and size evolution in caviomorph rodents. Biol J Linn Soc 121:907–922. https://doi.org/10.1093/biolinnean/blx026
Antoine PO, Marivaux L, Croft DA, Billet G, Ganerød M, Jaramillo C, Martin T, Orliac MJ, Tejada J, Altamirano A, Duranthon F, Fanjat G, Rousse S, Gismondi RS (2012) Middle Eocene rodents from Peruvian Amazonia reveal the pattern and timing of caviomorph origins and biogeography. Proc. Royal Soc. B 279:1319–1326. https://doi.org/10.1098/rspb.2011.1732
Arends A, McNab BK (2001) The comparative energetics of ‘caviomorph’ rodents. Comp Biochem Physiol A Mol Integr Physiol 130:105–122
Arnaudo ME, Arnal M (2023) First virtual endocast description of an early Miocene representative of Pan-Octodontoidea (Caviomorpha, Hystricognathi) and considerations on the early encephalic evolution in South American rodents. J Paleontol 97(2):454–476
Bapst DW (2012) Paleotree: an R package for paleontological and phylogenetic analyses of evolution. Methods Ecol Evo 3:803–807. https://doi.org/10.1111/j.2041-210X.2012.00223.x
Bertrand OC, Silcox MT (2016) First virtual endocasts of a fossil rodent: Ischyromys typus (Ischyromyidae, Oligocene) and brain evolution in rodents. J Vert Paleontol 36:1–19. https://doi.org/10.1080/02724634.2016.1095762
Bertrand OC, Silcox MT (2023) Brain evolution in fossil rodents: a starting point. In: Dozo MT, Paulina-Carabajal A, Macrini TE, Walsh S (eds) paleoneurology of amniotes: new directions in the study of fossil endocasts. Springer International Publishing, Cham, pp 645–680
Bertrand OC, Amador-Mughal F, Silcox MT (2016) Virtual endocasts of Eocene Paramys (Paramyinae): oldest endocranial record for Rodentia and early brain evolution in Euarchontoglires. Proc R Soc B 283:1–8. https://doi.org/10.1098/rspb.2015.2316
Bertrand OC, Amador-Mughal F, Silcox MT (2017) Virtual endocast of the early Oligocene Cedromus wilsoni (Cedromurinae) and brain evolution in squirrels. J Anat 230:128–151. https://doi.org/10.1111/joa.12537
Bertrand OC, Amador-Mughal F, Lang MM, Silcox MT (2018) Virtual endocasts of fossil Sciuroidea: brain size reduction in the evolution of fossoriality. Palaeontol 61:919–948. https://doi.org/10.1111/pala.12378
Bertrand OC, Püschel HP, Schwab JA, Silcox MT, Brusatte SL (2021) The impact of locomotion on the brain evolution of squirrels and close relatives. Commun Biol 4:460. https://doi.org/10.1038/s42003-021-01887-8
Blomberg SP, Garland T, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745. https://doi.org/10.1111/j.0014-3820.2003.tb00285.x
Burgin CJ, Colella JP, Kahn PL, Upham NS (2018) How many species of mammals are there? J Mammal 99:1–14. https://doi.org/10.1093/jmammal/gyz052
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35. https://doi.org/10.1007/s00265-010-1029-6
Collyer ML, Adams DC (2023) RRPP: linear model evaluation with randomized residuals in a permutation procedure. R package version 1.4.0. https://CRAN.R-project.org/package=RRPP
Dozo MT (1997a) Paleoneurología de Dolicavia minuscula (Rodentia, Caviidae) y Paedotherium insigne (Notoungulata, Hegetotheriidae) del Plioceno de Buenos Aires, Argentina. Ameghiniana 34:427–435
Dozo MT (1997b) Primer estudio paleoneurológico de un roedor caviomorfo (Cephalomyidae) y sus posibles implicancias filogenéticas. Mastozool Neotrop 4:89–96
Eisenberg J (1981) The mammalian radiations: an analysis of trends in evolution, adaptation, and behavior. University of Chicago Press, Chicago
Engelman RK (2022) Resizing the largest known extinct rodents (Caviomorpha: Dinomyidae, Neoepiblemidae) using occipital condyle width. R Soc Open Sci. https://doi.org/10.1098/rsos.220370
Fernández-Villoldo JA, Verzi DH, Lopes RT, Reis SF, Perez SI (2023) Brain size and shape diversification in a highly diverse South American clade of rodents (Echimyidae): a geometric morphometric and comparative phylogenetic approach. Bio J Linn Soc 140:227–295
Ferreira JD, Negri FR, Sánchez-Villagra MR, Kerber L (2020) Small within the largest: brain size and anatomy of the extinct Neoepiblema acreensis, a giant rodent from the Neotropics. Biol Lett 16:20190914. https://doi.org/10.1098/rsbl.2019.0914
Ferreira JD, Dozo MT, Bubadué J, Kerber L (2022) Morphology and postnatal ontogeny of the cranial endocast and paranasal sinuses of capybara (Hydrochoerus hydrochaeris), the largest living rodent. J Morphol 283:66–90. https://doi.org/10.1002/jmor.21428
Heldstab SA, Isler K, Graber SM, Schuppli C, Van Schaik CP (2022) The economics of brain size evolution in vertebrates. Curr Biol 32:R697–R708. https://doi.org/10.1016/j.cub.2022.04.096
Jerison HJ (1973) Evolution of the brain and intelligence. Academic Press
Kerber L, Ferreira JD, Negri FR (2019) A reassessment of the cranial morphology of Neoepiblema acreensis (Rodentia: Chinchilloidea), a Miocene rodent from South America. J Morphol 280:1821–1838. https://doi.org/10.1002/jmor.21067
Madozzo-Jaén MC (2019) Systematic and phylogeny of Prodolichotis prisca (Caviidae, Dolichotinae) from the Northwest of Argentina (late Miocene–early Pliocene): advances in the knowledge of the evolutionary history of maras. C R Palevol 18:33–50
Maugoust J, Orliac MJ (2023) Anatomical correlates and nomenclature of the chiropteran endocranial cast. Anat Rec 306:2791–2829
Millien V (2008) The largest among the smallest: the body mass of the giant rodent Josephoartigasia monesi. Proc R Soc B 275:1953–1955. https://doi.org/10.1098/rspb.2008.0087
Millien V, Bovy H (2010) When teeth and bones disagree: body mass estimation of a giant extinct rodent. J Mammal 91:11–18. https://doi.org/10.1644/08-MAMM-A-347R1.1
Mones A, Ojasti J (1986) Hydrochoerus hydrochaeris. Mamm Species 264:1–7
Ni X, Flynn JJ, Wyss AR, Zhang C (2019) Cranial endocast of a stem platyrrhine primate and ancestral brain conditions in anthropoids. Sci Adv 5:eaav7913. https://doi.org/10.1126/sciadv.aav7913
Nomina Anatomica Veterinaria (2017) International Committee on Veterinary Gross Anatomical Nomenclature, 6th edition.
Novacek MJ (1982) The brain of Leptictis dakotensis. an Oligocene leptictid. Eutheria Mammalia from North America. J Paleontol 56:1177–1186
Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W (2018) caper: comparative analyses of phylogenetics and evolution in R. R package version 1.0.1, https://CRAN.R-project.org/package=caper
Pascual R, Vucetich MG, Scillato-Yané GJ (1990) Extinct and recent South American and Caribbean Edentates and Rodents: outstanding examples of isolation. Atti Dei Convegni Lincei 87:627–640
Pérez ME, Vallejo-Pareja MC, Carrillo JD, Jaramillo C (2017) A new Pliocene capybara (Rodentia, Caviidae) from northern South America (Guajira, Colombia), and its implications for the Great American Biotic Interchange. J Mamm Evol 24:111–125. https://doi.org/10.1007/s10914-016-9356-7
Pilleri G (1959) Das Gehirn von Dolichotis patagona und Hydrochoerus hydrochaeris, nebst Betrachtungen uber die endocraniellen Verhältnisse (Rodentia, Hystricomorpha). Acta Zoologica 40:43–58
Pilleri G, Gihr M, Kraus C (1984) Cephalization in rodents with particular reference to the Canadian Beaver (Castor canadensisi). In: Pilleri G (ed) Investigations on Behavers. Brain Anatomy Institute, pp 11–102
Pinheiro J, Bates D, R Core Team (2022) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-160, https://CRAN.R-project.org/package=nlme
Rasia LL, Candela AM (2019) Upper molar morphology, homologies and evolutionary patterns of chinchilloid rodents (Mammalia, Caviomorpha). J Anat 234:50–65. https://doi.org/10.1111/joa.12895
Revell LJ (2012) phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. https://doi.org/10.1111/j.2041-210X.2011.00169.x
Rinderknecht A, Blanco RE (2008) The largest fossil rodent. Proc R Soc B275:923–928. https://doi.org/10.1098/rspb.2007.1645
Upham N, Patterson B (2015) Evolution of the caviomorph rodents: a complete phylogeny and time tree of living genera. In: Vassallo A, Antonucci D (eds) Biology of caviomorph rodents: diversity and evolution. Sociedad Argentina para el estudio de los Mamíferos, pp 63–120
Vucetich MG, Arnal M, Deschamps CM, Pérez ME, Vieytes EC (2015) A brief history of caviomorph rodents as told by the fossil record. In: Vassallo A, Antonucci D (eds) Biology of caviomorph rodents: Diversity and evolution. Sociedad Argentina Para el estudio de los Mamíferos, pp 11–62
Acknowledgements
We thank Alexandra Wegmann, Gabriel Aguirre Fernández, and Jorge D. Carrillo Briceño for helping us scan the specimens, and Lawrence Witmer (Ohio University), Digimorph.org, UTCT, Timothy Rowe, and Jeri Rodgers, for providing scans of caviomorph skulls. We especially thank the reviewers Mary Silcox and Ornella Bertrand for their comments which significantly improved previous versions of this manuscript.
Funding
JDF was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)—Finance Code 001. LFG was supported by Coordenação de Amparo à Pesquisa (CAPES 88882.345599/2019-01 and PrInt 88887.583738/2020-00). JMB is supported by a postdoc fellowship funded by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro—FAPERJ (E-26/204.166/2021 and E-26/204.167/2021), and by the American Society of Mammogists (2023 Oliver Pearson Award). MTD is supported by CONICET 2020 Executing Units Project awarded to the Patagonian Institute of Geology and Paleontology (PUE-IPGP 22920200100014). LK is supported Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 422568/2018-0; 309414/2019-9; 406902/2022-4.), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (21/2551-0002030-0), and CAPES (PrInt 8881.310240/2018-01).
Author information
Authors and Affiliations
Contributions
AR performed the scanning of Josephoartigasia’s cranium. LK MRSV and MTD were responsible for scanning comparative specimens. JDF performed the segmentation, 3D modeling, collection of quantitative data, body mass and encephalization estimates. LFG was responsible for gathering the ecological traits. JMB conducted all the statistical analyses. All the authors contributed to the study’s design and the manuscript’s drafting, provided final approval for publication, and agree to be held accountable for the work performed herein.
Corresponding author
Ethics declarations
Conflict of interest
We have no competing interests.
Ethics
We confirm that this work does not violate any ethical or legal aspects of paleontological research. All specimens were analyzed with the permission of the curators of the scientific collections mentioned in the text.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ferreira, J.D., Rinderknecht, A., de Moura Bubadué, J. et al. Unveiling the neuroanatomy of Josephoartigasia monesi and the evolution of encephalization in caviomorph rodents. Brain Struct Funct 229, 971–985 (2024). https://doi.org/10.1007/s00429-024-02762-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-024-02762-y