Abstract
The Subthalamic Nucleus (STh) is a lens-shaped subcortical structure located ventrally to the thalamus, that despite being embryologically derived from the diencephalon, is functionally implicated in the basal ganglia circuits. Because of this strict structural and functional relationship with the circuits of the basal ganglia, the STh is a current target for deep brain stimulation, a neurosurgical procedure employed to alleviate symptoms in movement disorders, such as Parkinson’s disease and dystonia. However, despite the great relevance of this structure for both basal ganglia physiology and pathology, the neurochemical and molecular anatomy of the STh remains largely unknown. Few studies have specifically addressed the detection of neurotransmitter systems and their receptors within the structure, and even fewer have investigated their topographical distribution. Here, we have reviewed the scientific literature on neurotransmitters relevant in the STh function of rodents, non-human primates and humans including glutamate, GABA, dopamine, serotonin, noradrenaline with particular focus on their subcellular, cellular and topographical distribution. Inter-species differences were highlighted to provide a framework for further research priorities, particularly in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Subthalamic Nucleus (STh) is a lens-shaped subcortical structure located ventrally to the thalamus, that despite being embryologically derived from the diencephalon, is functionally implicated in the basal ganglia circuits. Because of this strict structural and functional relationship with the circuits of the basal ganglia, and in particular the motor circuits mediating movement suppression, the STh is a current target for deep brain stimulation (DBS), a neurosurgical procedure employed to alleviate symptoms in movement disorders, particularly Parkinson’s disease (PD) and dystonia (Temel et al. 2006; Antonini et al. 2018; Deuschl et al. 2022).
A prominent model of the STh’s functional anatomy is known as the tripartite model (Parent and Hazrati 1996; Joel and Weiner 1997; Keuken et al. 2012; Lambert et al. 2015; Alkemade and Forstmann 2014). According to this morpho-functional subdivision of the STh, the anterior STh is functionally related to the limbic circuit, the dorsolateral STh is involved in the motor circuit, and the ventromedial STh is involved in the associative circuit of the basal ganglia. However, the anatomical segregation between the subdivisions of the STh is debated, with several studies suggesting significant overlap between functional territories, especially in humans (Keuken et al. 2012; Alkemade and Forstmann 2015; Lambert et al. 2015). While animal models present inherent advantages over human studies, interspecies differences, particularly between rodents, non-human primates and humans, represent a crucial point in the ongoing debate on STh functionality (Hardman et al. 2002; Baunetz et al. 2011). This is particularly relevant since DBS is performed only in human subjects with specific clinical inclusion criteria (Antonini et al. 2018; Deuschl et al. 2022) and human tracer studies are limited to ex-vivo slow-diffusing dyes (Emmi et al. 2020). Indeed, while the rodent STh presents an open structure with most dendritic arborizations extending into other subcortical regions, in primates, and particularly humans, dendritic fields in the STh are confined to the anatomical boundaries of the nucleus (Rafols and Fox 1976; Alkemade and Forstmann 2014). Aside of structural differences, basal ganglia circuits in rodents and primates also appear to differ in terms of segregation of functional loops, as well as in the role played by different nuclei (Aoki et al. 2019; Joel and Weiner 1997; Emmi et al. 2020).
Recent evidence in rodents (Aoki et al. 2019) indicates segregation of limbic, associative and motor circuits, even though an unidirectional influence of the limbic over the motor loop via the substantia nigra pars reticulata (SNr) has been discovered. In non-human primates and humans, segregation seems to be maintained with regard to the associative loops (associative regions being contacted exclusively by other associative regions), but not at the level of the motor loops (motor regions being contacted by other functional divisions, such as the limbic and associative; Joel and Weiner 1997). Moreover, there appears to be a progressive shift from the substantia nigra pars reticulata (SNr) (in rodents) to the internal Globus Pallidus (GPi) (in primates) in mediating basal ganglia outputs to the thalamus, with subsequent consequences on subthalamo-pallidal and subtalamo-nigral projections (Hardman et al. 2002; Emmi et al. 2020). Indeed, Kelly and Strick (2004) did not find evidence of retrograde labeled axons in the SNr, but only in the GPi, upon STh tracer injection in non-human primates.
To further underline this aspect, tracer studies on the cortico-subthalamic tract (or hyperdirect pathway) in non-human primates performed by Haynes and Haber (2013) indicate both functional specificity and functional integration of limbic, associative and motor afferences to the STh. This refers to the topographical segregation of cortico-subthalamic projections to specific STh regions, paired with the notion derived from Rafols and Fox (1976)’s findings on wide-spanning dendritic arborizations in STh neurons. Hence, while STh afferences may present a precise regional topography (Haynes and Haber 2013), dendritic arborizations of STh may traverse multiple functional territories and receive information from more than one functional loop. Considering the increasing importance of the STh in processing different types of information through phylogenesis (Hardman et al. 2002), the definition of the STh’s role in open versus closed loop circuits could represent an important aspect regarding information processing within the human basal ganglia.
Nevertheless, despite evidence indicating functional intergation of STh afferences and the presence of functional gradients as opposed to functional territories with distinct boundaries, definition of “predominantly” limbic, associative and motor areas of the STh remains crucial for DBS in treating neurological and psychiatric diseases (Temel et al. 2000; Antonini et al. 2018; Deuschl et al. 2022). Indeed, while one one side there appears to be a progressive drive in supporting functional intergation between limbic, associative and motor functions in the STh, reflecting complex information processesing occurring in humans, on the other there is an urgent yet unmet need of defining safe-to-target regions of the STh during DBS, with the ultimate goal to reduce unwanted side effects while maximizing treatment effectiveness, improving patient quality of life (Rodriguez-Rojas et al. 2022).
This macro- and meso-scale level of investigation, with predominant focus on anatomical connections and broad functional gradients, requires further integration with the functional microscopic anatomy of the nucleus at a regional, cellular and even subcellular level.
Indeed, the hypothesized functional subdivision of the STh allows for the definition of functional specializations also at the cellular and subcellular level. As functional specialization of single cells is defined throughout development, neurons migrate and partially segregate according to their molecular profile, forming distinct populations with possibly distinct functions (Arendt 2008; Alkemade et al. 2019). This represents the framework upon which identification of specific neuronal populations, defined by distinct neurochemical markers, can lead to the identification of functional territories in the STh.
Methodologically, the neurochemical and receptorial organization of the STh has been investigated post-mortem through the aid morphological methods, such as in-situ RNA labeling techniques (in-situ hybridization, ISH, and fluorescent ISH, or FISH), immunohistochemistry, immunofluorescence and autoradiography, allowing the identification of neuronal subpopulations, and thereby potential functional subdivisions within the structure. Information on receptor expression and distribution within the STh could provide significant advantages in defining functionally segregated regions, thus advancing our understanding of structure’s subdivision and circuitry.
While we have previously described the morphology, topography and connectivity of the STh in humans and non-human primates (Emmi et al. 2020), recent work by Alkemade et al. (2019) has focused on the characterization of the functional microscopic anatomy of the structure, with particular regard to the distribution of GABAergic, glutamatergic, dopaminergic and serotoninergic signaling markers.
Despite this, the neurochemical and molecular anatomy of the STh, i.e. the expression and topographical distribution of neurotransmitters and receptor proteins within the structure, remains controversial and largely unknown (Alkemade et al. 2019). Few studies have specifically addressed the expression of receptors within the STh, and even fewer have investigated their topographical distribution within the nucleus. Furthermore, most data are derived from rodent and non-human primate studies, with little to no information being available on humans for the different systems of neurotransmitters and signaling molecules.
Finally, of the studies investingating the neurochemical anatomy of the STh in humans, very few consider the three-dimensionality of the structure or examine it in its whole rostro-caudal extent.
Hence, the aim of this review is to assess available studies in the literature addressing the expression and distribution of receptors, neurotransmitters and signaling molecules of relevance within the STh, differentiating between data deriving from animal studies (rodents and non-human primates) and findings in humans. The ultimate objective of this study is to further underline the need to validate and translate the findings deriving from animal models to humans, despite known methodological and technical limitations.
Neurotransmitter systems and their receptors in the subthalamic nucleus
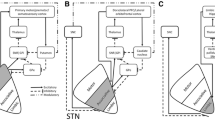
The following paragraphs describe the main neurotransmitter systems, the ionotropic and metabotropic receptors, as well as correlated structural and functional markers that are relevant for STh physiology and pathology. For each major system, a summary table and a schematic figure and is provided for quick orientation throughout included studies.
Glutamatergic system
Glutamatergic system markers have been extensively investigated in rodents, non-human primates and humans, with little-to-no interspecies differences, suggesting that the glutamatergic system maintains its organization throughout phylogenesis. Studies addressing glutamatergic system markers are reported in Table 1. Figure 1 displays known topographical, cellular and subcellular localizations of glutamatergic system markers based on the examined studies.
Glutamatergic system markers in the Subthalamic Nucleus (STh). Glutamate and its receptors represent the most characterized neurotransmitter system of the STh, with numerous studies addressing both its topography within the structure, as well as the cellular and subcellular localization of receptors. Graphical representations are based on the most recent available human and non-human primate studies, as reported in Table 1. In case of discordant findings, representation of marker topography was based on the most recent study addressing whole-volume distribution in the Subthalamic Nucleus
Glutamatergic neurons represent the most conspicuous neuronal population in the STh. Glutamatergic STh neurons are considered type 1 projection neurons and are homogeneously distributed throughout the STh in rodents, non-human primates and humans (Iwahori 1978; Albin et al. 1989; Parent and Hazrati 1995; Shink et al. 1996; Clarke et al. 1997; Feeley Kearney and Albin 1999; Wang et al. 2000; Tai et al. 2001; Lévesque and Parent 2005). Similarly, glutamatergic fibers, whether afferent or efferent, have been detected throughout the whole structure across different species. In the rodents,glutamate was detected in correspondence to asymmetrical synapses (axo-dendritic synapses) (Bevan et al. 1995; Clarke et al. 1997), in particular at the level of synaptic terminals arising from the cortex and the parafascicular thalamic nucleus and directed towards the STh. In humans, Alkemade et al. (2019) detected vescicular glutamate transporter 1 (VGLUT1) immunoreactivity as punctuate fiber labeling, with increased density at the borders of the STh.
Glutamatergic ionotropic receptors. Glutamatergic ionotropic receptors (iGluR) comprise AMPA, NMDA, and Kainate receptor families. In the rodent STh, AMPA and NMDA receptors (and their subunits), are predominantly localized at the level of glutamatergic and gabaergic asymmetrical synapses (Clarke et al. 1997). Similarly, diffuse expression of GluR 5/6/7 subunits and binding for Kainate receptors was detected diffusely troughout the whole STh. (Wüllner et al. 1997; Lobo et al. 2003). In primates, Wang et al. (2000) evidenced that AMPA receptor subunit GluR1 is present in STh neurons, and is expressed at the level of the soma and proximal dendrites. GluR2/3 subunits were also detected, but were less expressed than GluR1. According to the authors, this indicates that some of the postsynaptic AMPA receptors may consist in homomers of GluR1. NMDA receptors were present in both soma and dendrites. According to Klockgether et al. 1991, AMPA receptor expression in STh is quantitatively predominant when compared to NMDA receptors, even though local application of NMDA antagonists in the STh decreases the metabolic activity of the STh neurons (Nakanishi et al. 1988; Blandini et al. 2001).
In the human STh, Ball et al. (1994) documented diffuse expression of NMDA, AMPA and Kainate receptors throughout the whole STh. Studies on the anatomical localization of iGluR in the STh do not suggest inter-species differences, nor indicate a defined topography within the structure. Nevertheless, no information concerning the subcellular localization of iGluR receptor subunits is available in humans, which have been extensively investigated in rodents and non-human primates.
Glutamatergic metabotropic receptors. Metabotropic glutamatergic receptors (mGluR) are diffusely distributed throughout the STh in rodents (Fotuhi et al. 1993), even though their cellular and subcellular localization were not investigated. In non-human primates, mild expression of mGluR subunits was detected at the level of the cell body, while more prominent expression was evidenced at the level of the distal dendrites (Kuwajima et al. 2004). In particular, Kuwajima et al. (2004) mGluR1 and mGluR5 were concentrated mainly in the synaptic area in close proximity to GABA-ergic synapses; Furthermore, while ionotropic glutamate receptors (AMPA and NMDA) were expressed mainly at a post-synaptic level, mGluR were located presynaptically (Wang et al. 2000).
In humans, Phillips et al. (1999) reported diffuse immunoreactivity to mGluR2 without specific topography; the subcellular localization of mGluR was not investigated in humans.
These findings suggest that, as for iGluR, mGluR are diffusely distributed throughout the STh with no relevant interspecies differences.
GABAergic system
The STh receives prominent GABAergic afferences from the external segment of the Globus Pallidus (GPe) in rodents, non-human primates and humans (Smith and Parent 1988; Clarke et al. 1997; Emmi et al. 2020); In rodents and non-human primates, the majority of GABAergic pallidal terminals exhibited numerous varicosities, reminiscent of boutons en passant or boutons terminaux, forming synapses predominantly with proximal dendrites and less frequently with the soma and the distal dendrites (Parent and Hazrati 1995; Emmi et al. 2020). Other sources of GABAergic input to the STh, in rodents and non-human primates, include the pedunculopontine tegmental nucleus and the latero-dorsal tegmental nucleus (Usunoff et al. 2003). In humans, diffuse expression of GABA transporter 1 (GAT-1) documented by Augood et al. (1999) confirms that the STh is extensively innervated by GABAergic afferences. Recent evidence by Alkemade et al. (2019) confirmed moderate fiber terminal staining for Glutamic Acid Decarboxylase (GAD), which converts glutamate to GABA; presynaptic boutons were observed extending beyond the dorsolateral border of the STN, appearing as a cap on the dorsolateral tip of the nucleus.
While the presence of GABAergic terminals has been well established in all species, the presence of GABAergic neurons in the STh was initially controversial. Earlier studies in rodents (Yasumi et al. 1988) and non-human primates (Smith and Parent 1988) failed to identify GABAergic neurons in this structure, while later evidence identified GAD mRNA in STh cells across species (Levesque and Parent 2005), even though the neuronal nature of these cells, nor morphological features, were described. In humans, a study by Levesque and Parent (2005) evidenced that approximately 7% of the neurons within the human STh express GAD. The distribution of these neurons, which were morphologically identified as small Golgi type II interneurons, appears to follow an increasing dorsoventral gradient, with prominent density at the level of the ventral STh. According to the authors, the high density of GABAergic interneurons in the associative regions of the STh reflects the complex neural integration that underlies the anticipation, motivation and planning of movements which partakes within these territories. These findings are also supported by Alkemade et al. (2019) more recent study on humans. To date, no study to our knowledge has comparatively assessed GABAergic neurons across species, as previously performed by Hardman et al. (2002) for total neuronal populations. Hence, it is unclear whether the relative quantitiy, and topographic distribution, of GABAergic neurons in the STh differs across species.
GABAergic receptors
Since the STh receives prominent GABAergic innervation from the globus pallidus, as previously stated, GABA-A and GABA-B receptors are widely distributed within the whole STh of rodents and non-human primates (Charara et al. 2000, 2004; Schwarzer et al. 2001; Galvan et al. 2004). GABA-A receptor subunits are expressed by small bi- and tripolar neurons within the rodent STh (Schwarzer et al. 2001); a similar expression of GABA-A receptor subunits within the non-human primate STh was reported Kultas-Ilinsky, Leontiev and Whiting (1998). GABA-B receptor subunits 1 and 2 are reported to be homogeneously distributed throughout the whole extent of the rodent and non-human primate STh (Charara et al. 2000, 2004; Galvan et al. 2004).
In humans GABA-A receptor subunits have been described to follow a dorsolateral-ventromedial increasing gradient (Wu et al. 2018). The low immunoreactivity for GABA-A receptor subunits displayed by GAD-positive interneurons suggest that pallidal GABAergic projection neurons contact mainly glutamatergic projection neurons. Recent evidence by Alkemade et al. (2019) shows that GABA-A receptor subunit alpha 3 (GABRA3) is predominantly expressed in the neuronal soma, in addition to punctuate fiber staining.. Expression of GABA-B receptors in humans also appears to follow a similar dorsolateral-ventromedial increasing gradient of expression as GABA-A (Wu et al. 2018),
In the context of STh physiology, GABAergic afferences play a fundamental role in the modulation of the firing rate pattern of discharges and bursting activity. As seen above, GABAergic neurons in the STh do not appear to receive GABAergic afferences, which are mostly directed towards primary (glutamatergic) neurons. Furthermore, GAD + neurons do not express calcium binding proteins, such as parvalbumin, calbindin or calretinin, which are expressed in GAD− neurons. The increasing ventrolateral gradient of GABAergic interneurons evidenced by Lévesque and Parent (2005), coupled with the increasing dorsolateral to ventromedial gradient of expression of GABA-A and GABA-B receptors on primary neurons described by Wu et al. (2018), could reflect the functional specificity of this region, as associative areas generally contain more interneurons for the fine-tuning of the incoming signals. Nevertheless, the presence of topographically defined populations of GABAergic neurons, as well as the expression patterns of GABA receptors requires further investigation in non-human primates and rodents. In fact, it is yet unclear wheter GABAergic neuronal populations in the STh increase throughout phylogenesis, and if this phenomenon is limited to the ventromedial aspects of the nucleus, as seen in humans. Interspecies studies could provide useful information concerning the development of the STh throughout phylogenesis, clarifying whether local GABAergic interneuronal populations are related to the shift from segregated towards integrated circuits in the basal ganglia. Studies addressing GABAergic system markers are reported in Table 2. Figure 2 displays known topographical, cellular and subcellular localizations of GABAergic system markers based on the examined studies.
GABAergic system markers in the Subthalamic Nucleus (STh). GABAergic neurons (GAD +) represent a minor population of neurons within the STh, and are mainly located in the ventral aspects of the nucleus. An increasing dorsolateral to ventromedial gradient was found for the expression of GABA-a and GABA-b receptors. GABA-a receptors are found mainly on distal dendrites. Graphical representations are based on the most recent available human and non-human primate studies, as reported in Table 2. In case of discordant findings, representation of marker topography was based on the most recent study addressing whole-volume distribution in the Subthalamic Nucleus
Dopaminergic system
Dopaminergic afferences to the STh derive from the substantia nigra pars compacta (SNpc) (Emmi et al. 2020), even though the extent of dopaminergic innervation of the STh remains to be elucidated. In rodents, a conspicuous dopaminergic nigro-subthalamic bundle has been described (Hassani et al. 1997). Non-human primate studies demonstrated that labeled axons originating from the mediodorsal part of area A9 terminated mainly in the anteromedial STh, whereas those originating from area A8 terminated diffusely throghout the STh (François et al. 2000), suggesting a specific arrangement of dopaminergic fibers originating from the SNpc. Human studies, on the other hand, suggest prominent dopaminergic innervation of non-motor regions of the STh, particularly the anterior and ventromedial pole (Hedreen et al. 1999; Emmi et al. 2022). Diffuse projections throughout the whole STh are also reported in humans (Augood et al. 2000). Hedreen et al. (1999) evidenced that dopaminergic fibers present fine axonal branching patterns, compatible with terminal arborizations, only in the non-motor (containing calretinin + neurons) regions of the STh, while larger non-terminal axons could be detected in the motor areas. These findings suggest that dopaminergic innervation occurs at the level of the non-motor regions of the STh, while dopaminergic fibers travel across motor areas of the STh, likely directed towards the striatum, without forming synapses (Hedreen et al. 1999).
Dopaminergic receptors
Similar to dopaminergic innervation of the STh, the expression of dopaminergic receptors remains controversial and poorly understood, particularly in humans. The most studied receptors include dopaminergic receptor D1 and D2,, with few studies investigating other dopaminergic receptor families. In rodents, dopaminergic receptor D1 displayed variable immunoreactivity that ranged from absent to moderate across studies (Dawson et al. 1986; Dubois et al. 1986; Fremeau et al. 1991; Johnson et al. 1994). Only Savasta et al. (1986) reported a high expression of D1R whithin rodent STh, while Mansour et al. (1992) evidenced dense D1 receptor binding but no evidence of D1 mRNA. This suggest that while D1R proteins may be present, STh neurons do not express D1R mRNA; this uncoupling between protein immunoreactivity and mRNA expression generally suggests D1R protein expression in afferent axons targeting the STh, with the corresponding cell bodies expressing D1R mRNA being located elsewhere. Indeed, non-human primate studies detected presynaptic D1R on preterminal axons of putative glutamatergic and GABAergic terminals (Galvan et al 2014). Studies of the human STh reported no evidence for D1R expression (Augood et al. 2000; Hurd et al. 2001) D2R expression in rodent STh was reported as low by Dubois et al. (1986) and moderate by Johnson et al. (1994), without specific topogaphy. In non-human primates, D2 receptors were found presynaptically, on preterminal axons and putative glutamatergic and GABAergic terminals, similarly to what has been described for D1R(Galvan et al. 2014).
Studies on humans reported conflicting results as far as D2R is concerned, ranging from negative (Augood et al. 2000), to low (Hurd et al. 2001) or moderate (Wang et al. 2000), with little-to-no topographic information available. In our recent studies on the human STh, D2R was expressed predominantly at the level of the dendritic spines of β-III-tubulin positive neurites with a decreasing ventral to dorsal gradient (Emmi et al. 2022). Non-neuronal expression of D2R was also found, suggesting astrocytic expression of D2R. The expression of other dopaminergic receptors, such as D3R and D4R, was documented in humans by Wang et al. (2000) and Matsumoto et al. (2002) respectively, but their topographical distribution was not reported.
Several aspects concerning the dopaminergic system in the STh remain to be investigated. In particular, the extent and exact topography of dopaminergic afferences, and whether or not these present terminal arborizations rather than passing fibers, remains to be elucidated. This is particularly relevant, as small dopaminergic terminal axons may modulate the activity of the STh, with particular regard to the non-motor regions, which appear to be more prominently interested by this (Hedreen et al. 1999); the consequence of the dopaminergic deafferentation of the STh in PD is poorly understood, but it could participate in the hyperactivity of the STN observed in animal models of Parkinson's disease. Indeed, it has been demonstrated that the increased activity of STh neurons following midbrain dopaminergic lesion cannot be due solely to removal of pallido-subthalamic inhibition, and it has been suggested that the intrinsic dopaminergic innervation of the STN could also participate in its hyperactivity (François et al. 2000). Furthermore, the exact subcellular localization of dopaminergic receptors, whether pre- or post-synaptically, remains to be elucidated in humans, and may represent another relevant factor in determining STh alterations following dopaminergic denervation. Studies addressing dopaminergic system markers are reported in Table 3. Figure 3 displays known topographical, cellular and subcellular localizations of dopaminergic system markers based on the examined studies.
Dopaminergic system markers in the Subthalamic Nucleus (STh). Tyrosine Hydroxylase (TH) + fibers, deriving from the dopaminergic substantia nigra, are known to course ventrally to the STh and through the structure at the level of the ventromedial pole. The expression of dopaminergic receptors, in particular D1, D3, D4 and D5 is poorly known in humans. D2 receptors are found in a dorsolateral to ventromedial increasing gradient at the level of preterminal synapses and dendritic spines. Graphical representations are based on the most recent available human and non-human primate studies, as reported in Table 3. In case of discordant findings, representation of marker topography was based on the most recent study addressing whole-volume distribution in the Subthalamic Nucleus
Serotoninergic system (5-HT)
Serotoninergic (5-HT) innervation of the STh has been widely discussed in rodents, non-human primates and humans (Parent et al. 2011; Emmi et al., 2020). Across species, and particularly evident in non-human primates and humans, serotoninergic axons targeting the STh mostly derive from a single bundle of axons detaching from the main serotoninergic pathway, coursing in the lateral hypothalamic area and following the track of the lenticular fasciculus along the dorsal surface of the STh. A second smaller bundle is known to run along the ventral surface of the STh (Parent et al. 2011). In rodents, an increasing density of 5-HT immunoreactive fibers in the caudal part of the nucleus was detected (Steinbush et al. 1981), while also numerous terminal fibers were identified in the medial and ventral aspects of the STh (Mori et al. 1985). In non-human primates and humans, this is confirmed to occur particularly in the anteromedial and anterior-ventral aspects of the STh (Mori et al. 1985; Parent et al. 2011). Serotonin Transporter (SERT) positive fibers were described throughout the structure by both Martín-Cora and Pazos (2004) and Alkemade et al. 2019 in humans. Studies addressing 5-HT system markers are reported in Table 4. Figure 4 displays known topographical, cellular and subcellular localizations of 5-HT system markers based on the examined studies.
Serotoninergic and Cholinergic system markers in the Subthalamic Nucleus (STh). Serotonin receptors families were mainly investigated in rodents and non-human primates, and no topographical organization was reported in literature. The course of cholinergic fibers throughout the STh was investigated in rodents, and requires confirmation in humans. Nicotinic and muscarinic receptor expression was reported, but not their topography. Graphical representations are based on the most recent available human and non-human primate studies, as reported in Tables 4 and 5. In case of discordant findings, representation of marker topography was based on the most recent study addressing whole-volume distribution in the Subthalamic Nucleus (Table 6)
5-HT Receptors
The expression of 5-HT receptors within the rodent STh has been widely explored in literature, and also documented in primates and humans (Waeber et al. 1989). Reznitsky, et al. (2016)’s study did not detect 5-HT1a receptor mRNA and binding sites, while several studies reported the expression of 5-HT1b receptor in rodents (Bruinvels, Palacios and Hoyer 1993; Boschert et al. 1994; Reznitsky, Plenge and Hay-Schmidt 2016). Rodent STh was also reactive for 5-HT1c (Wright et al. 1995) and 5-HT1d receptors, the latter displaying a diffuse distribution and mild immunoreactivtiy (Bruinvels, Palacios and Hoyer 1993). Concerning 5-HT2 receptors, rodent STh was negative for 5-HT2a (Pompeiano, et al. 1994; Reznitsky et al. 2016), but positive for 5-HT2c (Pompeiano, et al. 1994; Eberle-Wang et al. 1997; Clemett et al. 2000; Reznitsky, et al. 2016). In particular, Pompeiano et al. (1994) and Eberle-Wang et al. (1997) evidenced high levels of 5-HT2c mRNA. 5-HT2c has also been detected in primates (López-Giménez et al. 2001). The STh was also positive for mRNA and binding sites for 5-HT4 in rodents (Vilaró, et al. 2005). Concerning the human STh, moderate binding for 5-HT7 receptor was detected thorugh autoradiography (Martín-Cora and Pazos 2004). Hence, while an anterio-medial and antero-ventral 5-HT innervation has been described across species, particularly evident in primates, the topography of 5-HT receptor families within the structure remains to be investigated, as the majority of studies available in literature have focused on rodents.
Cholinergic system (acetylcholine)
The cholinergic innervation of the STh appears to be uncertain and controversial. Even though the STh receives afferences from the pedunculopontine tegmental nucleus, which was believed to be an exclusively cholinergic nucleus, the recent discovery of consistent glutammatergic and dopaminergic neuronal populations within the PPT has questioned whether these connections are cholinergic or glutammatergic in nature (Marani et al. 2008). Choline acetyltransferase (ChAT) was detected in fibers coursing through the STh in rodents (Woolf and Butcher, 1986; Clarke et al. 1997; Kita and Kita 2011). These fibers originate from the peduncolopontine nucleus (Woolf and Butcher 1986) and travel through the STh from the caudodorsal to the rostroventral surface, giving off thin branches with small boutons (Kita and Kita 2011). The terminals of these fibers were enriched with glutamate, but not with GABA (Clarke et al. 1997). Studies on the porcine STh indicate prominent cholinergic innervation (Larsen et al. 2004). In humans, ChAT-rich thick and straight axons are reported to enter the STh dorsally and densely innervate the structure with rich terminal arborizations (Mesulam et al. 1992). Very finely varicose axons seemed to encircle subthalamic neurons, giving the appearance of a honeycomb pattern (Mesulam et al. 1992). The topography of these fibers, however, has not been investigated throughout the rostro-caudal extent of the structure.
Nicotinic and muscarinic receptors. In non-human primates, mild expression of nicotinic receptor α3, α4 and α7 subunits was reported, while β2 and β4 nicotinic receptor subunit expression was uncertain (Cimino et al. 1992; Quik et al. 2000). The non-human primate STh was not reactive for α6 and β3 subunits. Nicotinic receptors have been evidenced also in humans through autoradiography (Pimlott et al. 2004), and a moderate expression of α4 subunit mRNA was identified by Agulhon et al. (1998) within the fetal human STh. Low expression of muscarinic M2 receptors in the human STh was documented by Warren et al. (2007).Studies addressing cholinergic system markers are reported in Table 5. Figure 4 displays known topographical, cellular and subcellular localizations of cholinergic system markers based on the examined studies.
Noradrenergic sytem
Even though some authors evidenced projections arising from the locus coeruleus to the STh (Carpenter et al. 1981; Canteras et al. 1990), there are currently no studies available in literature addressing the noradrenergic innervation of the STh in humans. Conversely, dopamine-beta-hydroxylase (DβH) immunoreactive fibers have been evidenced in non-human primates (Ginsberg et al. 1993; Masilamoni, et al. 2017). Interestingly, MPTP-treated parkinsonian non-human primates present a significant decrease in DβH fiber density within the STh, indicating possible implications for PD (Masilamoni et al. 2017). However, the normal organization of these fibers, with particular regard to the topography of terminal arborizations, remains to be investigated.
Mild reactivity for adrenergic receptors α1 and α2 has been reported in rodents (Belujon et al. 2007). Studies addressing noradrenergic system markers are reported in Table 6.
Purinergic receptors
Very little evidence is available on the expression and distribution of purinergic receptors in the human and non-human primate basal ganglia, despite the recent interest in the purinergic modulation of basal ganglia circuitry and the approval of the first purinergic drug for the treatment of Parkinson’s Disease in the United States and Japan. The expression of adenosine receptor A1 within the human STh was described by Misgeld et al. (2007), while our group has described the expression and distribution of A2A receptors (Emmi et al. 2022). A2A receptors were detected as dot-like reactivities colocalizing predominantly with β-III-Tubulin positive neurites, with the exception of sporadic somatic reactivites and non-β-III-tubulin positive structures, likely glial cells, as previously reported by Pelassa et al. (2019). Topographically, A2AR were expressed according to a dorsal to ventral decreasing gradient within the human STh.
Purinergic receptor P2X2 was investigated in rodents by Kanjhan et al. (1999) by means of in-situ hybridization and immunohistochemistry, revealing diffuse immunoreactivity of the subthalamic nucleus. In humans, the P2Y(1) receptor was found to be prominently expressed in the neurons of the subthalamic nucleus (Moore et al. 2000).
Histaminergic system
Very little information concerning the histaminergic system in the STh is available in the literature. In rodents, marked Histamine Receptor 3 (H-3) mRNA expression was detected (Rouleau et al. 2004). In humans, binding to H-3R was investigated by means of autoradiography by Goodchild et al. (1999), but no detectable binding was found for the STh. Nevertheless, morphological investigation on histaminergic receptors is still scarce, and their expression in primates and humans remains to be confirmed.
Opioid receptors
The expression of µ receptor in rodents was reported by Han et al. (2018), with a high rate of coexpression with melanocortin receptor 4. Non-human primate STh was found to express µ, κ and δ opioid receptor mRNA, with higher µ receptor expressionin the ventral STh. Preproenkephalin-b mRNA was also evidenced in primates, and its expression was found to increase after levodopa tratment in dyskinetic monkeys (Aubert et al. 2007). The presence of opioid receptor in human STh was investigated throug RNA blotting, indentifying the expression of endogenous opioid receptors (Raynor et al. 1995). mRNA of µ receptor was detected in human STh neurons, but no clear cellular κ and δ receptor mRNA was reported (Peckys and Landwehrmeyer 1999). Unlike primates, humans appear to present differential expression of opioid receptor mRNA. However, these findings require further confirmation via immunohistochemistry. Studies addressing opioid system markers are reported in Table 7. Figure 5 displays known topographical, cellular and subcellular localizations of opioid system markers based on the examined studies.
Opioid and Cannabinoid system markers in the Subthalamic Nucleus (STh). While µ receptors present a ventral increasing gradient within the STh, the topography for the major opioid and cannabinoid receptor families was not investigated. However, coexpression of µ receptor and melanocortin receptor 4 is known to occur in a set of STh neurons. Graphical representations are based on the most recent available human and non-human primate studies, as reported in Tables 7 and 8. In case of discordant findings, representation of marker topography was based on the most recent study addressing whole-volume distribution in the Subthalamic Nucleus
Cannabinoid receptors
Cannabinoid receptor expression was found in the rodent STh (Mailleux and Vanderhaeghen 1992) but not in non-human primates, as reported by Marani et al. (2008). Tsou et al. (1998) detected CB1 mRNA, but not CB1 receptor proteins in the rodent STh; this likely suggests CB1 receptor protein localization on terminal efferent axons. Rojo-Bustamante et al. (2018) reported the expression of CB1 in the non-human primate STh through RT-PCR analyses. Cannabinoid receptor binding in the human STh was not detected via autoradiography (Glass, Faull and Dragunow 1997). Further studies on cannabinoid receptor expression in humans are necessary to comprehend the effects of this system on STh function. Studies addressing cannabinoid system markers are reported in Table 8. Figure 5 displays known topographical, cellular and subcellular localizations of cannabinoid system markers based on the examined studies.
Calcium channels and calcium-binding proteins
While the presence of calcium channels has been reported in rodents (see Marani et al. 2008), few authors have studied the distribution of calcium channels in the human STh. Monteil et al. (2000) evidenced the presence of the α1 subunit of calcium channels within the human STh, while Yang et al. (2014) indicate that the effects of DBS may be mediated by T-type calcium channels present on subthalamic neurons.
Calcium binding proteins have received substantial attention due to their involvement in calcium mediated signaling, often reflecting specific firing patterns in neurons. Studies on non-human primates have evidenced how specific calcium binding proteins, particularly calretinin, calbindin and parvalbumin can identify distinct neuronal populations within the brain, particularly the basal ganglia. Indeed, earlier evidence pointed towards calretinin as a distinct marker of non-motor STh neurons, being located predominantly at the level of the medial aspect of the structure (Fortin and Parent 1996). Marked neuronal immunoreactivity for Parvalbumin was also detected in non-human primates (Côté et al. 1991). In the human STh, a higher expression of calretinin following a dorsolateral-ventromedial increasing gradient has been evidenced, while parvalbumin is distributed according to a dorsolateral-ventromedial decreasing gradient (Morel et al. 2002; Lévesque and Parent 2005; Wu et al. 2018). Human STh neurons also displayed a moderate immunoreactivity to SMI-32 (Morel et al. 2002; Wu et al. 2018). Parent et al. (1996) and Augood et al. (1999) evidenced a specific topographic distribution of calcium-binding proteins within the human STh: in particular, calretinin was expressed mainly in neurons located in the ventromedial aspect of the nucleus, while parvalbumin was found mainly in neurons within the dorsolateral regions. However, even though a topographical organization was reported, significant overlap between territories was also evidenced. Furthermore, Alkemade et al. (2019) identified parvalbumin immunoreactivity at the level of cell bodies, as well as diffuse labeling of fibers, and calretinin labeled both cell bodies and fibers. Interestingly, Lévesque and Parent (2005) did not detect calcium binding protein expression in GAD + interneurons in the STh. Nevertheless, no studies available in literature have yet investigated the co-expression of different calcium binding proteins in human STh. While expression of parvalbumin and calretinin appears to indicate different functional gradients within the STh, boundaries between specific calcium-binding expressing territories are diffuse and do not allow for the identification of distinct STh regions. Studies addressing calcium-binding proteins and other system markers are reported in Table 9. Figure 6 displays known topographical, cellular and subcellular localizations of calcium-binding proteins based on the examined studies.
Calcium binding proteins in the Subthalamic Nucleus (STh). Calbindin is known to present an increasing dorsolateral to ventromedial gradient of expression, while Parvalbumin presents a diametrically opposite pattern, with an increasing gradient from the ventromedial to the dorsolateral pole. Graphical representations are based on the most recent available human and non-human primate studies, as reported in Table 9. In case of discordant findings, representation of marker topography was based on the most recent study addressing whole-volume distribution in the Subthalamic Nucleus
Discussion
In the present review, we have assessed morphological studies examining the expression and distribution of markers for different neurotransmitter systems in the STh of rodents, non-human primates and humans. No studies assessed in this review have defined a clear anatomical segregation of any of the investigated neurotransmitter systems; rather, these studies have evidenced variable distribution gradients of neurochemical markers throughout the main axes of the STh, particularly in humans. This seems to suggest that complete segregation of functional territories within the STh, as originally hypothesized with the tripartite hypothesis, should be considered a conceptual simplification of a much more complex and variable internal organization of the STh (Alkemade and Forstmann 2014), with often overlapping functional territories (Emmi et al. 2020). Moreover, while the debate has previously stressed the dicotomy between functional segregation versus functional integration/convergence, revision of previous studies (Keuken et al. 2012) and recent evidence in primates (Haynes and Haber 2013; Emmi et al. 2020) indicates both functional specificity and integration. Indeed, non-human primate dendritic arborizations in the STh span across the main axis of the nucleus and occupy over two-thirds of its volume (Rafols and Fox 1976); hence, STh dendritic arborizations likely stretch across multiple functional regions, suggesting convergence between inputs from different functional areas, which appear greater than what appears based on projection patterns (Haynes and Haber 2013). Indeed, STh neurons at the center of a functional region may also receive inputs from functionally diverse cortical areas onto more distal dendrites (Bevan et al. 1997). Therefore, the output from each subthalamic neuron, although primarily driven by the cortical input matching the territory in which the neuron lies, is likely to result from the integration of functionally diverse information. This overlap between STh territories appears to increase throughout phylogenesis, with non-human primates and humans presenting little to no defined boundaries between hypothesized functional territories, possibly reflecting integration of limbic, associative and motor functions within the basal ganglia circuitry in these species (Hardman et al. 2002; Alkemade and Forstmann 2014). How this integration occurs at cellular, subcellular and molecular levels remains yet to be clarified, particularly in humans. To our knowledge, no studies in literature have yet examined the three-dimensional morphological characteristics of human STh neurons (for example, through silver impregnation techniques), and the notion of wide-spanning dendritic arborizations, likely receiving information projected to different STh regions, is mutuated from non-human primate studies (Rafols and Fox 1976) and remains to be confirmed in humans. Moreover, the expression of neurochemical markers of different neurotransmitter systems, and in particular different receptor families mediating the effects of incoming STh afferences, have not always been systematically assessed in humans. Older studies investigating neurotransmitter systems throughout the whole brain often do not consider the whole-rostro caudal extent of the STh, limiting observation to a single section of the nucleus. Moreover, unless high-magnification and thorough observation of multiple sections is performed, smaller immunoreactivities can be eaisly overlooked, as seen for dopaminergic terminal axons (Hedreen et al. 1999; François et al. 2000). This is particularly relevant for specific neurotransmitter systems, rather than others. Indeed, while glutamatergic and GABAergic systems have been more thoroughly investigated in humans, revealing a defined topographical organization mainly for GABAergic neurons and GABA receptor families in the STh, other neurotransmitters, like the dopaminergic, noradrenergic, serotoninergic and purinergic systems, have not received significant attention in humans, with at times contradicting results. Among others, dopaminergic receptors families are particularly relevant to STh function, given the known dopaminergic projections it receives from the substantia nigra (Hedreen et al. 1999; Alkemade et al. 2019; Emmi et al. 2022). We have recently evidenced how, at the level of the subthalamic nucleus, dopaminergic receptors D2 colocalize with purinergic receptors A2a, suggesting that these receptors form heteromeric receptor mosaiques (Emmi et al. 2022), and that receptor-receptor interactions occur also in the STh, as previously demonstrated for the striatum (Fernández-Dueñas et al. 2019). This notion also opens up to investigation of other receptor-receptor interactions in the STh, not only in neuronal cells, but also in glial cells; indeed, D2–A2a receptor heterodimers were discovered in the astrocytic processes of the striatum (Pelassa et al. 2019), and are potentially involved in Parkinson’s Disease pathophysiology. Similarly, we evidenced expression of A2a and D2 receptors in non-neuronal cells of the STh, even though further characterization, and more specific assays for detecting receptor-receptor interactions (i.e. proximity ligation assay, PLA), are required. Nevertheless, the possibility of investigating receptor-receptor interactions, in both neuronal and non-neuronal cells, could be of potential interest for further defining functional gradients within the STh. Indeed, while these functional gradients were traditionally defined by evaluating markers on neuronal cells and, in rare cases, their three-dimensional topography, investigating non-neuronal cells in the STh and the expression of specific glio- and neurotransmitter systems in non-neuronal cells could provide novel insights in the functional anatomy of the STh.
Future perspectives
We believe future research on the functional anatomy of the STh should be performed by considering the most recent findings on co-occurring functional segregation and integration in the STh. Numerous older studies did not account for STh variability across the three-dimensional planes, mostly because this was beyond the scope of the study itself. STh specific studies performed on serial sections (Levesqué and Parent 2005), and the subsequent three-dimensional reconstruction of the nucleus in its whole extent (Alkemade et al. 2019; Emmi et al. 2021; 2022), are therefore highly warranted. Furthermore, we believe that, based on the available scientific literature, neurotransmitter systems and their receptors, such as the dopaminergic, serotoninergic, purinergic, and also cannabinoid and opioid systems, should receive more attention and further characterization in humans. Aside from individual receptors and their localization, the notion of receptor-receptor interactions and the detection of receptor mosaiques in the basal ganglia, greatly encourage the investigation of these phenomena also in the STh. Moreover, while research on the functional anatomy of the STh has predominantly focused on neurons, non-neuronal cells, such as astrocytes, have been characterized as potential major players in basal ganglia physiology and Parkinson’s Disease pathology; similar functional implications can be hypothesized for other nuclei, such as the STh. Hence, investigation of functional systems should also be extended to glial cells. Aside of morphological methods, other approaches have been employed to characterize the functional anatomy of the STh, with both advantages and limitations over histology. DBS itself has emerged as an intriguing tool to study functional aspects related to the STh (Aloisami et al. 2022), with the advantage of characterizing the electrophysiological properties of human neuronal populations in-vivo. Yet, this does not provide structural confirmation concerning the expression and distribution of specific neurochemical markers within the nucleus. Also, while DBS can be employed to study the electrophysiological properties of the STh, it must be noted that it is performed in humans only in case of pathology (PD, dystonia, etc.), and does not provide information concerning the physiological activity of STh neurons in this species; moreover, while electrophysiological recording of neuronal populations within the STh via DBS may suggest the involvement of specific neurotransmitters, or the expression of specific cell-membrane receptors, confirmation via morphological methods is required and should be integrated with this technique.
Lastly, while Parkinson’s Disease and other synucleinopathies generally do not affect the STh, at least via evident neuropathological alterations like Lewy bodies and neurites, functional alterations are well known to occur. The abnormal firing pattern of STh neurons in Parkinson patients is most-often regarded as a consequence of broader circuit perturbations occurring due to dopamine loss and nigral degeneration; the role of direct dopaminergic modulation, via nigro-subthalamic pathways, is often neglected (Emmi et al. 2020). Indeed, dopaminergic terminals have been detected in specific STh regions, and their loss has been reported in PD patients (Hedreen et al. 1999; François et al. 2000), likely playing a role in STh hyperactivity. While structural data suggest prominent dopaminergic innervation of non-motor areas, it is currently unknown how dopaminergic deafferentation affects functional regions of the STh, and whether or not this accounts for Parkinsonian symptoms (Antonini et al. 2023).
Conclusions
In conclusion, available structural data concerning the functional and neurochemical anatomy of the human STh is scarce, and studies from the literature, with few exceptions, are often descriptive in nature or do not report on the distribution of the markers within the whole extent of the structure. Moreover, there are very few studies assessing the extent of functional reorganization within the STh occurring as consequence of neurodegenerative diseases, like Parkinson’s Disease.
We hope this review of literature encourages future studies on the functional and neurochemical anatomy of the human STh, and that appropriate methodological approaches will be employed to evaluate the spatial distribution of relevant markers throughout the whole nucleus.
Data availability
All data are available by the corresponding author upon request.
References
Agulhon C et al (1998) Distribution of mRNA for the α4 subunit of the nicotinic acetylcholine receptor in the human fetal brain. Mol Brain Res 58(1–2):123–131. https://doi.org/10.1016/S0169-328X(98)00113-2
Albin RL et al (1989) Feline subthalamic nucleus neurons contain glutamate-like but not GABA-like or glycine-like immunoreactivity. Brain Res 491(1):185–188. https://doi.org/10.1016/0006-8993(89)90103-0
Alkemade A, Forstmann BU (2014) Do we need to revise the tripartite subdivision hypothesis of the human subthalamic nucleus (STN)? Neuroimage 95:326–329. https://doi.org/10.1016/j.neuroimage.2014.03.010
Alkemade A, Schnitzler A, Forstmann BU (2015) Topographic organization of the human and non-human primate subthalamic nucleus. Brain Struct Funct 220(6):3075–3086. https://doi.org/10.1007/s00429-015-1047-2
Alkemade A, de Hollander G, Miletic S, Keuken MC, Balesar R, de Boer O, Swaab DF, Forstmann BU (2019) The functional microscopic neuroanatomy of the human subthalamic nucleus. Brain Struct Funct 224(9):3213–3227. https://doi.org/10.1007/s00429-019-01960-3
Antonini A, Moro E, Godeiro C, Reichmann H (2018) Medical and surgical management of advanced Parkinson’s disease. Mov Disord 33(6):900–908. https://doi.org/10.1002/mds.27340
Antonini A, Emmi A, Campagnolo M (2023) Beyond the dopaminergic system: lessons learned from l-dopa resistant symptoms in Parkinson’s disease. In press, Movement Disorders Clinical Practice
Aoki S, Smith JB, Li H, Yan X, Igarashi M, Coulon P et al (2019) An open cortico-basal ganglia loop allows limbic control over motor output via the nigrothalamic pathway. Elife 8:e49995. https://doi.org/10.7554/eLife.49995
Arendt D (2008) The evolution of cell types in animals: emerging principles from molecular studies. Nat Rev Genet 9(11):868–882. https://doi.org/10.1038/nrg2416
Aubert I et al (2007) Enhanced preproenkephalin-B–Derived opioid transmission in striatum and subthalamic nucleus converges upon globus pallidus internalis in l-3,4-dihydroxyphenylalanine–Induced dyskinesia. Biol Psychiat 61(7):836–844. https://doi.org/10.1016/j.biopsych.2006.06.038
Augood S et al (1999) Localization of calcium-binding proteins and GABA transporter (GAT-1) messenger RNA in the human subthalamic nucleus. Neuroscience 88(2):521–534. https://doi.org/10.1016/S0306-4522(98)00226-7
Augood, S. J. et al. (2000) ‘Localization of dopaminergic markers in the human subthalamic nucleus.’, The Journal of comparative neurology, 421(2): 247–55. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10813785.
Ball EF et al (1994) The distribution of excitatory amino acid receptors in the normal human midbrain and basal ganglia with implications for Parkinson’s disease: a quantitative autoradiographic study using [3H]MK-801, [3H]glycine, [3H]CNQX and [3H]kainate. Brain Res 658(1–2):209–218. https://doi.org/10.1016/S0006-8993(09)90028-2
Baunez C, Yelnik J, Mallet L (2011) Six questions on the subthalamic nucleus: lessons from animal models and from stimulated patients. Neuroscience 198:193–204. https://doi.org/10.1016/j.neuroscience.2011.09.059
Belujon P, Bezard E, Taupignon A, Bioulac B, Benazzouz A (2007) Noradrenergic modulation of subthalamic nucleus activity: behavioral and electrophysiological evidence in intact and 6-hydroxydopamine-lesioned rats. J Neurosci 27(36):9595–9606. https://doi.org/10.1523/JNEUROSCI.2583-07.2007
Bevan MD, Francis CM, Bolam JP (1995) The glutamate-enriched cortical and thalamic input to neurons in the subthalamic nucleus of the rat: convergence with GABA-positive terminals. J Comp Neurol 361(3):491–511. https://doi.org/10.1002/cne.903610312
Bevan MD, Clarke NP, Bolam JP (1997) Synaptic integration of functionally diverse pallidal information in the entopeduncular nucleus and subthalamic nucleus in the rat. J Neurosci 17(1):308–324. https://doi.org/10.1523/JNEUROSCI.17-01-00308.1997
Blandini F, Nappi G, Greenamyre JT (2001) Subthalamic infusion of an NMDA antagonist prevents basal ganglia metabolic changes and nigral degeneration in a rodent model of Parkinson’s disease. Ann Neurol 49(4):525–529. https://doi.org/10.1002/ana.104
Boschert U et al (1994) The mouse 5-hydroxytryptamine 1B receptor is localized predominantly on axon terminals. Neuroscience 58(1):167–182. https://doi.org/10.1016/0306-4522(94)90164-3
Bruinvels AT, Palacios JM, Hoyer D (1993) Autoradiographic characterisation and localisation of 5-HT1D compared to 5-HT1B binding sites in rat brain. Naunyn-Schmiedeberg’s Arch Pharmacol 347(6):569–582. https://doi.org/10.1007/BF00166939
Canteras NS et al (1990) Afferent connections of the subthalamic nucleus: a combined retrograde and anterograde horseradish peroxidase study in the rat. Brain Res 513(1):43–59. https://doi.org/10.1016/0006-8993(90)91087-W
Carpenter MB, Carleton SC et al (1981) Connections of the subthalamic nucleus in the monkey. Brain Res 224(1):1–29. https://doi.org/10.1016/0006-8993(81)91113-6
Charara A et al (2000) Pre- and postsynaptic localization of GABA(B) receptors in the basal ganglia in monkeys. Neuroscience 95(1):127–140. https://doi.org/10.1016/S0306-4522(99)00409-1
Charara A et al (2004) An electron microscope immunocytochemical study of GABAB R2 receptors in the monkey basal ganglia: a comparative analysis with GABAB R1 receptor distribution. J Comp Neurol 476(1):65–79. https://doi.org/10.1002/cne.20210
Cimino M et al (1992) Distribution of nicotinic receptors in cynomolgus monkey brain and ganglia: Localization of α3 subunit mRNA, α-bungarotoxin and nicotine binding sites. Neuroscience 51(1):77–86. https://doi.org/10.1016/0306-4522(92)90472-E
Clarke N et al (1997) Glutamate-enriched cholinergic synaptic terminals in the entopeduncular nucleus and subthalamic nucleus of the rat. Neuroscience 81(2):371–385. https://doi.org/10.1016/S0306-4522(97)00247-9
Clemett DA et al (2000) Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology 39(1):123–132. https://doi.org/10.1016/S0028-3908(99)00086-6
Dawson T et al (1986) D-1 dopamine receptors in the rat brain: a quantitative autoradiographic analysis. J Neurosci 6(8):2352–2365. https://doi.org/10.1523/JNEUROSCI.06-08-02352.1986
Deuschl G et al (2022) European academy of neurology/movement disorder society-European section guideline on the treatment of Parkinson’s disease: I. invasive therapies. Mov Disord 37(7):1360–1374. https://doi.org/10.1002/mds.29066
Dubois A et al (1986) Autoradiographic distribution of the D1 agonist [3H]SKF 38393, in the rat brain and spinal cord. Comparison with the distribution of D2 dopamine receptors. Neuroscience 19(1):125–137. https://doi.org/10.1016/0306-4522(86)90010-2
Eberle-Wang K et al (1997) ‘Pattern of expression of the serotonin2C receptor messenger RNA in the basal ganglia of adult rats.’, The Journal of comparative neurology, 384 (2): 233–47. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9215720.
Emmi A et al (2020) ‘Anatomy and Connectivity of the Subthalamic Nucleus in Humans and Non-human Primates’, Frontiers in Neuroanatomy, 14(April). doi: https://doi.org/10.3389/fnana.2020.00013.
Emmi A, Porzionato A, Contran M, De Rose E, Macchi V, De Caro R (2021) 3D reconstruction of the morpho-functional topography of the human vagal trigone. Front Neuroanat 15:663399. https://doi.org/10.3389/fnana.2021.663399
Emmi A, Antonini A, Sandre M, Baldo A, Contran M, Macchi V, Guidolin D, Porzionato A, De Caro R (2022) Topography and distribution of adenosine A2A and dopamine D2 receptors in the human Subthalamic Nucleus. Front Neurosci 16:945574. https://doi.org/10.3389/fnins.2022.945574
Feeley Kearney JA, Albin RL (1999) Intrasubthalamic nucleus metabotropic glutamate receptor activation: a behavioral, Fos immunohistochemical and [14C]2-deoxyglucose autoradiographic study. Neuroscience 95(2):409–416. https://doi.org/10.1016/S0306-4522(99)00439-X
Fernández-Dueñas V, Gómez-Soler M, Valle-León M, Watanabe M, Ferrer I, Ciruela F (2019) Revealing adenosine A(2A)-dopamine D(2) receptor heteromers in Parkinson’s disease post-mortem brain through a new alphascreen-based assay. Int J Mol Sci 20(14):3600. https://doi.org/10.3390/ijms20143600
Fortin M, Parent A (1996) Calretinin as a marker of specific neuronal subsets in primate substantia nigra and subthalamic nucleus. Brain Res 708(1–2):201–204. https://doi.org/10.1016/0006-8993(95)01374-1
Fotuhi M et al (1993) Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J Neurosci 13(5):2001–2012. https://doi.org/10.1523/JNEUROSCI.13-05-02001.1993
François C et al (2000) Dopaminergic innervation of the subthalamic nucleus in the normal state, in MPTP-treated monkeys, and in Parkinson’s disease patients. J Comp Neurol 425(1):121–129. https://doi.org/10.1002/1096-9861(20000911)425:1%3c121::AID-CNE10%3e3.0.CO;2-G
Fremeau RT et al (1991) ‘Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc National Academy Sci 88(9):3772–3776. https://doi.org/10.1073/pnas.88.9.3772
Galvan A et al (2004) Differential subcellular and subsynaptic distribution of GABAA and GABAB receptors in the monkey subthalamic nucleus. Neuroscience 127(3):709–721. https://doi.org/10.1016/j.neuroscience.2004.05.014
Galvan A et al (2014) Localization and function of dopamine receptors in the subthalamic nucleus of normal and parkinsonian monkeys. J Neurophysiol 112(2):467–479. https://doi.org/10.1152/jn.00849.2013
Ginsberg SD et al (1993) Noradrenergic innervation of the hypothalamus of rhesus monkeys: Distribution of dopamine-?-hydroxylase immunoreactive fibers and quantitative analysis of varicosities in the paraventricular nucleus. J Comp Neurol 327(4):597–611. https://doi.org/10.1002/cne.903270410
Glass M, Faull RL, Dragunow M (1997) Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77(2):299–318. https://doi.org/10.1016/S0306-4522(96)00428-9
Goodchild RE, Court JA, Hobson I, Piggott MA, Perry RH, Ince P, Jaros E, Perry EK (1999) Distribution of histamine H3-receptor binding in the normal human basal ganglia: comparison with Huntington’s and Parkinson’s disease cases. Eur J Neurosci 11(2):449–456. https://doi.org/10.1046/j.1460-9568.1999.00453.x
Han D-J, He Z-G , Yang H (2018) ‘Melanocortin-4 receptor in subthalamic nucleus is involved in the modulation of nociception.’, American journal of clinical and experimental immunology, 7(4): 76–80. Available at: http://www.ncbi.nlm.nih.gov/pubmed/30245921.
Hardman CD et al (2002) Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: Volume and neuronal number for the output, internal relay, and striatal modulating nuclei. J Comp Neurol 445(3):238–255. https://doi.org/10.1002/cne.10165
Hassani OK, François C, Yelnik J, Féger J (1997) Evidence for a dopaminergic innervation of the subthalamic nucleus in the rat. Brain Res 749(1):88–94. https://doi.org/10.1016/s0006-8993(96)01167-5
Haynes WI, Haber SN (2013) The organization of the prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for basal ganglia models and deep brain stimulation. J Neurosci 33:4804–4814. https://doi.org/10.1523/JNEUROSCI.4674-12.2013
Hedreen JC (1999) Tyrosine hydroxylase-immunoreactive elements in the human globus pallidus and subthalamic nucleus. J Comp Neurol 409(3):400–410. https://doi.org/10.1002/(SICI)1096-9861(19990705)409:3
Hurd YL, Suzuki M, Sedvall GC (2001) D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J Chem Neuroanat 22(1–2):127–137. https://doi.org/10.1016/S0891-0618(01)00122-3
Iwahori N (1978) A golgi study on the subthalamic nucleus of the cat. J Comp Neurol 182(3):383–397. https://doi.org/10.1002/cne.901820303
Joel D, Weiner I (1997) The connections of the primate subthalamic nucleus: indirect pathways and the open-interconnected scheme of basal ganglia-thalamocortical circuitry. Brain Res Brain Res Rev 23:62–78. https://doi.org/10.1016/s0165-0173(96)00018-5
Johnson AE et al (1994) Characterization of dopamine receptor binding sites in the subthalamic nucleus. NeuroReport 5(14):1836–1838. https://doi.org/10.1097/00001756-199409080-00038
Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF (1999) Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J Comp Neurol 407(1):11–32
Kelly RM, Strick PL (2004) Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res 143:449–459
Keuken MC et al (2012) Are there three subdivisions in the primate subthalamic nucleus? Front Neuroanat. https://doi.org/10.3389/fnana.2012.00014
Kita T, Kita H (2011) Cholinergic and non-cholinergic mesopontine tegmental neurons projecting to the subthalamic nucleus in the rat. Eur J Neurosci 33(3):433–443. https://doi.org/10.1111/j.1460-9568.2010.07537.x
Klockgether T et al (1991) The AMPA receptor antagonist NBQX has antiparkinsonian effects in monoamine-depleted rats and MPTP-treated monkeys. Ann Neurol 30(5):717–723. https://doi.org/10.1002/ana.410300513
Kultas-Ilinsky K, Leontiev V, Whiting P (1998) Expression of 10 GABAA receptor subunit messenger RNAs in the motor-related thalamic nuclei and basal ganglia of Macaca mulatta studied with in situ hybridization histochemistry. Neuroscience 85(1):179–204. https://doi.org/10.1016/S0306-4522(97)00634-9
Kuwajima M et al (2004) Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the monkey subthalamic nucleus. J Comp Neurol 474(4):589–602. https://doi.org/10.1002/cne.20158
Lambert C, Zrinzo L, Nagy Z, Lutti A, Hariz M, Foltynie T et al (2015) Do we need to revise the tripartite subdivision hypothesis of the human subthalamic nucleus (STN)? Response to Alkemade and Forstmann. Neuroimage 110:1–2. https://doi.org/10.1016/j.neuroimage.2015.01.038
Lévesque J-C, Parent A (2005) GABAergic interneurons in human subthalamic nucleus. Mov Disord 20(5):574–584. https://doi.org/10.1002/mds.20374
Lobo MK et al (2003) Ionotropic glutamate receptor expression and dopaminergic modulation in the developing subthalamic nucleus of the rat: an immunohistochemical and electrophysiological analysis. Dev Neurosci 25(6):384–393. https://doi.org/10.1159/000075664
López-Giménez JF et al (2001) Regional distribution and cellular localization of 5-HT 2C receptor mRNA in monkey brain: comparison with [3 H]mesulergine binding sites and choline acetyltransferase mRNA. Synapse 42(1):12–26. https://doi.org/10.1002/syn.1095
Mailleux P, Vanderhaeghen J-J (1992) Localization of cannabinoid receptor in the human developing and adult basal ganglia. Higher levels in the striatonigral neurons. Neurosci Lett 148(1–2):173–176. https://doi.org/10.1016/0304-3940(92)90832-R
Mansour A et al (1992) A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience 46(4):959–971. https://doi.org/10.1016/0306-4522(92)90197-A
Marani E et al (2008) ‘The subthalamic nucleus. Part I: development, cytology, topography and connections.’, Advances in anatomy, embryology, and cell biology, 198: 1–113, vii. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18727483.
Martín-Cora FJ, Pazos A (2004) Autoradiographic distribution of 5-HT 7 receptors in the human brain using [3 H]mesulergine: comparison to other mammalian species. Br J Pharmacol 141(1):92–104. https://doi.org/10.1038/sj.bjp.0705576
Masilamoni GJ, Groover O, Smith Y (2017) Reduced noradrenergic innervation of ventral midbrain dopaminergic cell groups and the subthalamic nucleus in MPTP-treated parkinsonian monkeys. Neurobiol Dis 100:9–18. https://doi.org/10.1016/j.nbd.2016.12.025
Matsumoto M et al (2002) Low Levels of mRNA for Dopamine D4 Receptor in Human Cerebral Cortex and Striatum. J Neurochem 66(3):915–919. https://doi.org/10.1046/j.1471-4159.1996.66030915.x
Misgeld U, Drew G, Yanovsky Y (2007) Presynaptic modulation of GABA release in the basal ganglia. Prog Brain Res. https://doi.org/10.1016/S0079-6123(06)60014-9
Monteil A et al (2000) Specific properties of T-type calcium channels generated by the human α1I subunit. J Biol Chem 275(22):16530–16535. https://doi.org/10.1074/jbc.C000090200
Moore D, Chambers J, Waldvogel H, Faull R, Emson P (2000) Regional and cellular distribution of the P2Y(1) purinergic receptor in the human brain: striking neuronal localisation. J Comp Neurol 421(3):374–384. https://doi.org/10.1002/(sici)1096-9861(20000605)421:3%3c374::aid-cne6%3e3.0.co;2-z
Morel A et al (2002) Neurochemical organization of the human basal ganglia: Anatomofunctional territories defined by the distributions of calcium-binding proteins and SMI-32. J Comparat Neurol 443(1):86–103. https://doi.org/10.1002/cne.10096
Mori S et al (1985) Immunohistochemical demonstration of serotonin nerve fibers in the subthalamic nucleus of the rat, cat and monkey. Neurosci Lett 62(3):305–309. https://doi.org/10.1016/0304-3940(85)90566-X
Nakanishi H, Kita H, Kitai ST (1988) An receptor mediated excitatory postsynaptic potential evoked in subthalamic neurons in an in vitro slice preparation of the rat. Neurosci Lett 95(1–3):130–136. https://doi.org/10.1016/0304-3940(88)90645-3
Parent A, Hazrati L-N (1995) Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidium in basal ganglia circuitry. Brain Res Rev 20(1):128–154. https://doi.org/10.1016/0165-0173(94)00008-D
Parent A et al (1996) Calcium-binding proteins in primate basal ganglia. Neurosci Res 25(4):309–334. https://doi.org/10.1016/0168-0102(96)01065-6
Parent M et al (2011) Serotonin innervation of basal ganglia in monkeys and humans. J Chem Neuroanat 41(4):256–265. https://doi.org/10.1016/j.jchemneu.2011.04.005
Peckys D, Landwehrmeyer GB (1999) Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience 88(4):1093–1135. https://doi.org/10.1016/s0306-4522(98)00251-6
Pelassa S, Guidolin D, Venturini A, Averna M, Frumento G, Campanini L et al (2019) A2A–D2 heteromers on striatal astrocytes: biochemical and biophysical evidence. Int J Mol Sci 20(10):2457. https://doi.org/10.3390/ijms20102457
Phillips T et al (1999) LocaliZation of metabotropic glutamate receptor type 2 in the human brain. Neuroscience 95(4):1139–1156. https://doi.org/10.1016/S0306-4522(99)00353-X
Pimlott SL et al (2004) Nicotinic acetylcholine receptor distribution in Alzheimer’s disease, dementia with Lewy bodies, Parkinson’s disease, and vascular dementia. In vitro binding study using 5-[125I]-A-85380. Neuropsychopharmacology 29(1):108–116. https://doi.org/10.1038/sj.npp.1300302
Pompeiano M, Palacios JM, Mengod G (1994) Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Mol Brain Res 23(1–2):163–178. https://doi.org/10.1016/0169-328X(94)90223-2
Quik M et al (2000) Localization of nicotinic receptor subunit mRNAs in monkey brain by in situ hybridization. J Comp Neurol 425(1):58–69. https://doi.org/10.1002/1096-9861(20000911)425:1%3c58::AID-CNE6%3e3.0.CO;2-X
Rafols JA, Fox CA (1976) The neurons in the primate subthalamic nucleus: a Golgi and electron microscopic study. J Comp Neurol 168:75–112
Raynor K et al (1995) ‘Characterization of the cloned human mu opioid receptor.’, The Journal of pharmacology and experimental therapeutics, 272(1), pp. 423–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7815359.
Reznitsky M, Plenge P, Hay-Schmidt A (2016) Serotonergic projections from the raphe nuclei to the subthalamic nucleus; a retrograde- and anterograde neuronal tracing study. Neurosci Lett 612:172–177. https://doi.org/10.1016/j.neulet.2015.11.035
Rodriguez-Rojas R et al (2022) Functional topography of the human subthalamic nucleus: relevance for subthalamotomy in Parkinson’s disease. Mov Disord 37(2):279–290. https://doi.org/10.1002/mds.28862
Rojo-Bustamante E et al (2018) The expression of cannabinoid type 1 receptor and 2-arachidonoyl glycerol synthesizing/degrading enzymes is altered in basal ganglia during the active phase of levodopa-induced dyskinesia. Neurobiol Dis 118:64–75. https://doi.org/10.1016/j.nbd.2018.06.019
Rouleau A, Héron A, Cochois V, Pillot C, Schwartz JC, Arrang JM (2004) Cloning and expression of the mouse histamine H3 receptor: evidence for multiple isoforms. J Neurochem 90(6):1331–1338. https://doi.org/10.1111/j.1471-4159.2004.02606.x
Savasta M, Dubois A, Scatton B (1986) Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H]SCH 23390. Brain Res 375(2):291–301. https://doi.org/10.1016/0006-8993(86)90749-3
Schwarzer C et al (2001) Distribution of the major ?-aminobutyric acidA receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. J Comp Neurol 433(4):526–549. https://doi.org/10.1002/cne.1158
Shink E et al (1996) The subthalamic nucleus and the external pallidum: two tightly interconnected structures that control the output of the basal ganglia in the monkey. Neuroscience 73(2):335–357. https://doi.org/10.1016/0306-4522(96)00022-X
Smith Y, Parent A (1988) Neurons of the subthalamic nucleus in primates display glutamate but not GABA immunoreactivity. Brain Res 453(1–2):353–356. https://doi.org/10.1016/0006-8993(88)90177-1
Steinbusch HWM (1981) Distribution of serotonin-immunoreactivity in the central nervous system of the rat—cell bodies and terminals. Neuroscience 6(4):557–618. https://doi.org/10.1016/0306-4522(81)90146-9
Tai LS et al (2001) Co-localization of AMPA-type glutamate receptor immunoreactivity in neurons of the rat subthalamic nucleus. Brain Res 895(1–2):95–103. https://doi.org/10.1016/S0006-8993(01)02036-4
Temel Y et al (2006) Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review. Parkinsonism Relat Disord 12(5):265–272. https://doi.org/10.1016/j.parkreldis.2006.01.004
Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83(2):393–411. https://doi.org/10.1016/s0306-4522(97)00436-3
Usunoff KG, Dimitar EI, Stephan RL, Andreas W (2003) ’Pedunculopontine tegmental nucleus Part I: cytoarchitecture, transmitters, development and connections. Biomed Rev 14:95–120
Vilaró MT, Cortés R, Mengod G (2005) Serotonin 5-HT 4 receptors and their mRNAs in rat and guinea pig brain: distribution and effects of neurotoxic lesions. J Comparat Neurol 484(4):418–439. https://doi.org/10.1002/cne.20447
Waeber C et al (1989) ‘5.HT1 receptors in the vertebrate brain.’ Naunyn-Schmiedeberg’s Arch Pharmacol. https://doi.org/10.1007/BF00260602
Wang XS et al (2000) ‘A light and electron microscopic study of glutamate receptors in the monkey subthalamic nucleus. J Neurocytol 29(10):743–754. https://doi.org/10.1023/a:1010990404833
Warren NM et al (2007) The basal ganglia cholinergic neurochemistry of progressive supranuclear palsy and other neurodegenerative diseases. J Neurol Neurosurg Psychiatry 78(6):571–575. https://doi.org/10.1136/jnnp.2006.099937
Woolf NJ, Butcher LL (1986) Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull 16(5):603–637. https://doi.org/10.1016/0361-9230(86)90134-6
Wright DE et al (1995) Comparative localization of serotonin1A, 1C, and2 receptor subtype mRNAs in rat brain. J Comp Neurol 351(3):357–373. https://doi.org/10.1002/cne.903510304
Wu XH et al (2018) GABA A and GABA B receptor subunit localization on neurochemically identified neurons of the human subthalamic nucleus. J Comparat Neurol 526(5):803–823. https://doi.org/10.1002/cne.24368
Wüllner U, Standaert DG, Testa CM, Penney JB, Young AB (1997) Differential expression of kainate receptors in the basal ganglia of the developing and adult rat brain. Brain Res 768(1–2):215–223. https://doi.org/10.1016/s0006-8993(97)00645-8
Yang Y-C et al (2014) The T-type calcium channel as a new therapeutic target for Parkinson’s disease. Pflügers Arch Eur J Physiol 466(4):747–755. https://doi.org/10.1007/s00424-014-1466-6
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
AE performed the literature search and revision. AE, AP, AA, RDC wrote the main manuscript text. AE prepared the figures and tables. All authors reviewed and contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no coonflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emmi, A., Campagnolo, M., Stocco, E. et al. Neurotransmitter and receptor systems in the subthalamic nucleus. Brain Struct Funct 228, 1595–1617 (2023). https://doi.org/10.1007/s00429-023-02678-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-023-02678-z