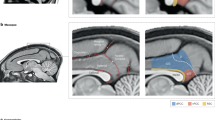
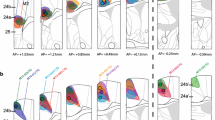
Abstract
The rabbit cingulate cortex is highly differentiated in contrast to rodents and numerous recent advances suggest the rabbit area map needs revision. Immunohistochemistry was used to assess cytoarchitecture with neuron-specific nuclear binding protein (NeuN) and neurocytology with intermediate neurofilament proteins, parvalbumin and glutamic acid decarboxylase. Key findings include: (1) Anterior cingulate cortex (ACC) area 32 has dorsal and ventral divisions. (2) Area 33 is part of ACC. (3) Midcingulate cortex (MCC) has anterior and posterior divisions and this was verified with extensive quantitative analysis and a horizontal series of sections. (4) NeuN, also known as Fox-3, is not limited to somata and formed nodules, granular clusters and striations in the apical dendrites of pyramidal neurons. (5) Area 30 forms a complex of anterior and posterior parts with further medial and lateral divisions. (6) Area 29b has two divisions and occupies substantially more volume than in rat. (7) Area 29a begins with a subsplenial component and extends relatively further caudal than in rat. As similar areal designations are often used among species, direct comparisons were made of rabbit areas with those in rat and monkey. The dichotomy of MCC is of particular interest to studies of pain as anterior MCC is most frequently activated in human acute pain studies and the rabbit can be used to study this subregion. Finally, the area 30 complex is not primarily dysgranular as in rat and is more differentiated than in any other mammal including human. The large and highly differentiated rabbit cingulate cortex provides a unique model for assessing cingulate cortex, pain processing and RNA splicing functions.


















Similar content being viewed by others
Abbreviations
- A30C:
-
Area 30 complex
- ACC:
-
Anterior cingulate cortex
- AD:
-
Anterodorsal thalamic nucleus
- aMCC:
-
Anterior MCC
- BSA:
-
Bovine serum albumin
- GAD:
-
Glutamic acid decarboxylase
- MCC:
-
Midcingulate cortex
- NeuN:
-
Neuron-specific nuclear binding protein
- NFP:
-
Non-phosphorylated intermediate neurofilament H proteins
- PBS:
-
Phosphate-buffered saline
- pMCC:
-
Posterior MCC
- PSF:
-
Polypyrimidine tract binding protein associated splicing factor
- PV:
-
Parvalbumin
- RSC:
-
Retrosplenial cortex
- RSG:
-
Retrosplenial granular cortex
References
Ballantine HT, Cassidy WL, Flanagan NB, Marino R Jr (1967) Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg 26:488–495
Braak H (1979) Pigment architecture of the human telencephalic cortex IV. Regio retrosplenialis. Cell Tissue Res 204:431–440
Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth, Leipzig
Buchanan SL, Powell DA, Buggy J (1984) H-2-Deoxyglucose uptake after electrical stimulation of cardiovascular sites in anterior medial cortex in rabbits. Brain Res Bull 13:371–382
Buchanan SL, Powell DA, Thompson RH (1989) Prefrontal projections to the medial nuclei of the dorsal thalamus in the rabbit. Neurosci Lett 106:55–59
Buchanan SL, Thompson RH, Maxwell BL, Powell DA (1994) Efferentconnections of the medial prefrontal cortex in the rabbit. Exp Brain Res 100:469–483
Farrell MJ, Laird AR, Egan GF (2005) Brain activity associated with painfully hot stimuli applied to the upper limb: a meta-analysis. Hum Brain Map 25:129–139
Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ (2005) Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492:145–177
Hof PR, Nimchinsky EA, Morrison JH (1995) Neurochemical phenotype of corticocortical connections in the macaque monkey—Quantitative analysis of a subset of neurofilament protein immunoreactive projection neurons in frontal, parietal, temporal and cingulate cortices. J Comp Neurol 362:109–133
Kim KK, Adelstein RS, Kawamoto S (2009) Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the fox-1 gene family of splicing factors. J Biol Chem 284:31052–31061
Kim KK, Kim YC, Adelstein RS, Kawamoto S (2011) Fox-3 and PSF interact to activate neural cell-specific alternative splicing. Nucleic Acids Res 39(8):3064–3078. doi:10.1093/nar/gkq1221
Lukoyanov NV, Lukoyanova EA (2006) Retrosplenial cortex lesions impair acquisition of active avoidance while sparing fear-based emotional memory. Behav Brain Res 173:229–236
Mullen RJ, Buck CR, Smith AM (1992) NeuN, a neuronal specific nuclear protein in vertebrates. Development 116:201–211
Nimchinsky EA, Hof PR, Young WG, Morrison JH (1996) Neurochemical, morphologic, and laminar characterization of cortical projection neurons in the cingulate motor areas of the macaque monkey. J Comp Neurol 374:136–160
Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, Nichelli P (2002) Does anticipation of pain affect cortical nociceptive systems? J Neurosci 22:3206–3214
Porro CA, Cettolo V, Francescato MP, Baraldib P (2003) Functional activity mapping of the mesial hemispheric wall during anticipation of pain. Neuroimage 19:1738–1747
Shibata H, Honda Y (2012) Thalamocortical projections of the anterodorsal thalamic nucleus in the rabbit. J Comp Neurol 520:2647–2656
Shibata H, Honda Y (2015) Thalamocortical projections of the anteroventral thalamic nucleus in the rabbit. J Comp Neurol 523(5):726–741
Sripanidkulchai K, Wyss JM (1986) Thalamic projections to the retrosplenial cortex in the rat. J Comp Neurol 254:143–165
Sripanidkulchai K, Wyss JM (1987) The laminar organization of efferent neuronal cell bodies in the retrosplenial granular cortex. Brain Res 406:255–269
Talairach J, Bancaud J, Geier S, Bordas-Ferrer M, Bonis A, Szikla G (1973) The cingulate gyrus and human behavior. Electroencephalogr Clin Neurophysiol 34:45–52
Vann SD, Aggleton JP (2005) Selective dysgranular retrosplenial cortex lesions in rats disrupt allocentric performance of the radial-arm maze task. Behav Neurosci 119:1682–1686
Vertes RP, Hoover WB (2008) Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol 508:212–237
Vogt BA (1993) Structural organization of cingulate cortex: areas, neurons, and somatodendritic transmitter receptors. In: Vogt BA, Gabriel M (eds) Neurobiology of cingulate cortex and limbic thalamus. Birkhauser, Boston, pp 19–70
Vogt BA (2005) Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6:533–544
Vogt BA (2015) Cingulate cortex and pain architecture. In: Paxinos G (ed) The rat nervous system, vol 4. Elsevier, Amsterdam
Vogt BA, Paxinos G (2014) Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct Funct 219:185–192
Vogt BA, Sikes RW (2009) Cingulate nociceptive circuitry and roles in pain processing: the Cingulate Premotor Pain Model. In: Vogt BA (ed) Cingulate neurobiology and disease. Oxford University Press, London
Vogt BA, Sikes RW, Swadlow HA, Weyand TG (1986) Rabbit cingulate cortex: cytoarchitecture, physiological border with visual cortex and afferent cortical connections including those of visual, motor, postsubicular and transcingulate origin. J Comp Neurol 248:74–94
Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR (1995) Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J Comp Neurol 359(3):490–506
Vogt BA, Vogt LJ, Perl DP, Hof PR (2001) Cytology of human caudomedial cingulate, retrosplenial, and caudal parahippocampal cortices. J Comp Neurol 438:353–376
Vogt BA, Vogt LJ, Farber NB, Bush G (2005) Architecture and neurocytology of monkey cingulate gyrus. J Comp Neurol 485(3):218–239
Vogt BA, Mohlberg H, Zilles K, Amunts K, Palomero-Gallagher N (2016) Human retrosplenial cortex: cytoarchitecture and probability maps. In preparation
Wiesendanger R, Wiesendanger M (1982) The corticopontine system in the rat: I. Mapping of corticopontine neurons. J Comp Neurol 208:215–226
Zhang L, Zhao Z-Q (2010) Plasticity changes of neuronal activities in central lateral nucleus by stimulation of the anterior cingulate cortex in rat. Brain Res Bull 81:574–578
Acknowledgments
I thank Dr. Robert W. Sikes and Leslie J. Vogt for assisting with the horizontally sectioned cases. This research was supported by Cingulum Neurosciences Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Vogt, B.A. Cytoarchitecture and neurocytology of rabbit cingulate cortex. Brain Struct Funct 221, 3571–3589 (2016). https://doi.org/10.1007/s00429-015-1120-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-015-1120-x