Abstract
Nowadays pathology laboratories are worldwide facing a digital revolution, with an increasing number of institutions adopting digital pathology (DP) and whole slide imaging solutions. Despite indeed providing novel and helpful advantages, embracing a whole DP workflow is still challenging, especially for wide healthcare networks. The Azienda Zero of the Veneto Italian region has begun a process of a fully digital transformation of an integrated network of 12 hospitals producing nearly 3 million slides per year. In the present article, we describe the planning stages and the operative phases needed to support such a disruptive transition, along with the initial preliminary results emerging from the project. The ultimate goal of the DP program in the Veneto Italian region is to improve patients’ clinical care through a safe and standardized process, encompassing a total digital management of pathology samples, easy file sharing with experienced colleagues, and automatic support by artificial intelligence tools.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the era of targeted medicine, pathologists must deal with an increasing daily workload to assess extremely detailed diagnoses to guide specific patient-designed therapies. In this scenario, digital pathology (DP) and whole-slide imaging (WSI) represent disruptive technologies with great potential to meet the needs of the modern medical community. These technologies not only allow pathologists to quickly share challenging cases with experienced, remotely located colleagues to get second opinions but they also enable scanned slides to be stored in virtual archives from where they may be easily and quickly retrieved [1]. Furthermore, DP and WSI perfectly suit the application of artificial intelligence (AI)-based algorithms, which can improve the accuracy of diagnosis and the development of personalized treatment plans, assisting clinicians with decision-making. Also, AI can provide healthcare systems across multiple locations with the fundamental process of slide screening to recognize morphological patterns to quickly guide proper diagnosis and therapy [2]; however, despite great strides in recent years, the adoption of DP remains limited to only a few pathology laboratories. While generally exploited for research purposes [3, 4], DP has also been progressively employed for primary diagnoses by several institutions [5, 6]. Such innovative tools have been variably adopted either by pathology laboratories alone [7] or by complex healthcare networks of hospitals [8], like as in South Tyrol, Italy [9], and the DigiPatICS project in Catalonia, Spain [10]. These networks, however, have found that connecting an extensive network of hospitals is a huge challenge. Having a group of modern but different hospitals sharing a unique model may be complex in terms of identifying proper hardware (scanners and displays etc.) and software tools for each laboratory. Furthermore, despite technological progress increasing the application of DP and its affordability, the pathology community’s widespread adoption of this technology for primary diagnosis is still lagging behind [11], hampered by technical and managerial issues [12]. These considerations are of particular concern for specific fields, such as cytopathology, which still lack broadly acknowledged validating guidelines [13].
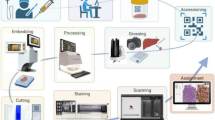
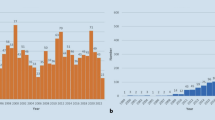
Azienda Zero is the broadest public healthcare provider in Veneto’s North Italian region and almost 5 million people benefit from the Azienda Zero healthcare system. It is organized into two leading academic hospitals (The University of Verona and Padova), a comprehensive cancer center/research (“Istituto di Ricovero e Cura a Carattere Scientifico”) hospital (“Istituto Oncologico Veneto;” IOV), and nine hospital networks (“Unità Locale Socio Sanitaria;” ULSSs); these latter networks include 26 smaller local districts. As far as pathological anatomy is concerned, the nine ULSSs, the two academic hospitals, and the IOV collect samples from institutions located throughout the entire Veneto territory [14]. While all of them are equipped with basic facilities, such as intraoperative frozen-section microscopy, a few hospitals provide more specific services. For example, (i) pathological samples from specialist surgery are analyzed by the laboratories of the two academic hospitals and three community hospitals; (ii) the Padova University Hospital and seven ULSSs gather cytological material from the whole region for the cervical cancer screening program, and (iii) an on-call pathologist is available 24/7 in the two academic hospitals and two community hospitals for intraoperative evaluation of transplantation specimens. The routine workflow of such laboratories globally accounts for about 240,000 slides per month and nearly 3 million slides per year (Fig. 1). In light of the above, the pathology laboratories in the Veneto region suit the adoption of a widely shared DP system. The aim of the present work is to describe the planning and effort required to enable the network of pathology laboratories in Veneto to go fully digital. In the following sections, we detail the characteristics of the hardware and software components considered and related to clinical needs, describing this crucial step of the digital transformation journey for pathological anatomy, providing a structured approach to assess feasibility, ensure compliance, and optimize the implementation of DP in the healthcare domain.
Context and methods
The DP program was supported with European funds to optimize pathological diagnosis in the network of public hospitals in the Veneto region. After a comprehensive assessment of data and needs provided by individual hospitals, the regional governance outlined a strategic framework with specific clinical needs. A market investigation called “Technologies for Digital Pathology” was carried out to verify the presence of appropriate tools tailored to the network’s needs. We want to stress that although technological tools, such as scanners and displays, represent one of the main points of the “digital pathway,” going fully digital is not only about buying tools; the transformation of a conventional pathology laboratory should encompass all the processes and the people routinely working there, who must be adequately trained to embrace the cultural change. Considering this step as mandatory for each laboratory, in the following sections, we describe the characteristics of the items necessary to realize the project according to the clinical needs.
Image-acquisition system
Scanners are essential for the digital workflow, and a variety of models are now available on the market [15]. Consultations with various suppliers were planned according to the predicted services for each community and academic hospital. Four scanner categories were selected based on their specific technical characteristics:
-
Batch 1: high-throughput scanners. Such devices were meant to satisfy the needs for routine diagnosis in high-volume institutions (i.e., the academic hospitals of Verona and Padova, and the community hospitals of Treviso, Venezia, and Vicenza) provided by high-volume specialistic surgical services. Therefore, these had to be capable of quickly and accurately digitizing a large number of hematoxylin and eosin (H&E)-stained slides and immunohistochemistry (IHC) preparations.
-
Batch 2: medium-throughput scanners. For the smaller community hospitals, there would have been no point in acquiring expensive hardware, performing far beyond the required daily workflow. Instead, these hospitals were more likely to benefit from less-expensive medium-capacity scanners, proficiently supplying the demands of their inpatient and outpatient services.
-
Batch 3: low-throughput scanners. This type of scanner was intended to address the two critical tasks of pathology laboratories: fluorescent microscopy and on-site intraoperative examination, both required for transplantation surgery. Such services would not have benefited from the bulky and heavy scanners commonly employed for routine diagnosis, as they usually deal with only a small number of slides. Conversely, light (but still fast) devices better suit these applications. For transplantation, quick digitization also allows physicians to share challenging cases with remotely located colleagues, which can help them to address the pressing demands of the operating theater.
-
Batch 4: cytopathology. Digitalization of cytological material always carries some issues linked to the peculiar characteristics of these specimens. Compared to histology, cytological material does not often homogeneously distribute onto slides, so scanning of multiple focal planes (“Z-stacking”) is often required to obtain appropriate WSIs [16]. The Z-stacking process typically results in longer scanning times and larger digital files. To overcome this problem, some companies have incorporated enhanced methods of Z-stacking into their products, for example, software selecting and combining the sharpest image from each focus into one single image (“Extended Focus”). As screening cytology accounts for a noteworthy percentage of the daily workflow of different hospitals, specific consultations were carried out to identify the most suitable devices, potentially integrating with already available AI-based support systems [17].
Workstation and viewing software
Digital displays (i.e., monitors) are an indispensable component of the workflow, and choosing the best display is just as important as selecting the most appropriate microscopy setup for a pathologist providing formal diagnoses. Displays vary greatly in parameters such as size, shape, esthetic design, resolution, brightness, contrast ratio, refresh rate, screen reflection, and viewing angle. A total of 230 workstations were used to let pathologists, residents, students, other professionals, and meeting attendants visualize and navigate WSIs. Each workstation was made up of a personal computer provided with a CPU i7 processor, 16 Gb RAM, 512 Gb SSD, and a full HD graphic card connected to two diagnostic medical-grade US Food and Drug Administration-approved full HD monitors (resolution 1920 × 1080 pixels), at least 27 inches in size. Users could use one display to show histological images and the other to view data. Regardless of the use case, there is a paucity of data on display specifications, particularly their definitions, how they apply to different display categories, and how the specifications can be used to help choose the right display for the intended use. Based on the tasks that they were intended for [18], we identified two categories of displays, distilled from manufacturer and computing hardware websites: (i) medical grade (MG) and (ii) consumer off-the-shelf (COTS). MG displays are typically expensive, built for multiyear use, and have standardized features to provide a uniform experience for their users. These are contrasted with COTS displays, which are general‐purpose displays. Most pathologists today use COTS displays as their primary display, provided to them as part of a standard core workstation configuration. Once scanned, pathologists can analyze displayed WSIs thanks to specific viewing software provided by the supplier, coupled with an internal laboratory integrative system (LIS). Apart from visualizing virtual slides, the viewing software allows physicians to navigate WSIs with a range of annotation functions, including drawing regions of interest, zooming in and out, rotating, and measuring.
Laboratory integrative system (Fig. 2)
One of the key points for the realization of the project was the unification of the workflow for all laboratories, which were thought of as a coordinated network. The distribution of pathology laboratories across multiple locations, with two high-volume academic hospitals, greatly suits this project. Therefore, to achieve this goal and following a market consultation, the PATHOXWEB 2.0 software provided by TESI Group was chosen as the regional LIS to be uniformly shared by all the institutions. PATHOXWEB 2.0 is a state-of-the-art updated web-based software developed to support professionals in handling the whole workflow of pathology laboratories going fully digital, from sample registration to definitive diagnosis and archiving. The web-based configuration of the system allows its remote usage on workstations and computers by any physician, technician, biologist, or other professional. Thanks to a middleware program, the software can be easily interfaced with local healthcare informatics systems and, as for the interests of a DP laboratory, has the following functions [19]:
-
Acceptance of requests, check-in, and registration of samples collected from inpatient and outpatient services by reading unique barcodes and assignment of a unique identifier for the accepted exam;
-
Assistance in specimen traceability and workflow quality checks in all laboratory phases. The software can be interfaced to laboratory devices that carry out sample tracking, including cassette printers, scanners, tissue processors, stainers, and gross-picture acquisition programs;
-
Residual material, blocks, and slide storage and retrieval management thanks to the interaction with specifically configured archiving desks;
-
Interface with WSI viewing software for supporting the visualization of scanned digital slides;
-
Assisted reporting with archiving of standard sentences and use of International Collaboration on Cancer Reporting-based checklists;
-
TelePathox program, a web-based consultation service allowing online correspondence of challenging cases with remotely located, experienced colleagues to get trusted second opinions.
Storage
According to the guidelines, “tissue blocks and glass slides should be kept long enough to ensure that the patient is treated properly”. Such a period has been operatively quantified by the UK’s Royal College of Pathologists as 10 years for histology slides and smears, and the patient’s entire life for blocks [20]. Planning a storage solution for digital files requires the consideration of several factors, including the number and size of the files, the number of users who will access the files, the interface between users, the level of data protection and security, and the budget. In the context of the realization of the current project, the digital storage process of WSI focused on the following points:
-
Cloud-based archiving of digital images and data in central/federated vendor-neutral archives, allowing easy and fast case identification and retrieval, planning for a long-term central archive after initial local management at the laboratory (short-term local archive). The purpose of this distinction is to streamline the management of the digital archive through centralization. It could be possible to automatically recover the slides of each patient every time there is an examination or letting the local pathologist decide which cases to keep in the short-term archive;
-
Association of digitally saved data with other healthcare-related information uploaded from the internal LIS;
-
Image backup option, able to provide disaster recovery and continuity exchange with external health organizations;
-
Indexing research tool employing the topography and morphology Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) vocabulary.
Artificial intelligence
The academic community of the Veneto region has always welcomed the adoption of new technologies as long as validated safety requirements support them. In pathology, as in many other scientific fields, AI is revolutionizing the approach to historically problematic issues, speeding up the decision-making process, and increasing the standardization of reports. Thus, with the goal of both enhancing diagnostic accuracy as well as reducing subjective interpretations and turn-around times, the present project chose to fully embrace the currently approved AI-based deep-learning tools, including:
-
Support for prostate biopsy screening for cancer and Gleason score grading [21];
-
Automatic evaluation of breast cancer IHC biomarkers (estrogen receptors, progesterone receptors, and Ki-67 proliferative index) [22];
-
Computer-assisted PD-L1 scoring in lung cancer specimens [23].
The use of the abovementioned products for in vitro diagnostic purposes has been endorsed by international regulatory entities after strict evaluation of their evidence-supported safety standards and performances.
Specialist networks
Specialist networks act to exchange knowledge, experiences and best practices, and training. In Veneto, the nephropathology network consists of numerous points of care that, however, cannot have the necessary expertise due to their diagnostic complexity. The project plans to structure reporting workstations within the network’s nephrology departments, similar to those in the pathology laboratories. This will allow the entire regional nephrology network to be connected, to manage the most complex cases, carry out process quality controls, standardize reporting, and consequently improving the therapeutic management of patients.
System tracking, quality checks, and archiving of tissue specimens
Standardized tracking, archiving, and conservation of materials are pivotal to patient care and may significantly influence diagnostic accuracy and, ultimately, clinical management. To ensure precision and repeatability of the whole workflow, pathology laboratories involved in the project had several quality control checkpoints. Barcode-scanning equipment integrated with a local LIS was employed to provide the lowest rate possible of human-eye-caused manual mistakes. First, each specimen received by the laboratory was accompanied by an e-request providing relevant clinical data created in local electronic healthcare record systems by the sending clinicians. After registration, the sample was given a unique identification number labeled with a DataMatrix-type barcode. Such an identification code is essential to track the specimen through its entire “life” within the laboratory, notifying professionals about its status, and eventual actions required from the registration to the final released diagnosis. Specifically, (i) labels were applied to the containers for sampling and material storing; (ii) barcodes were printed on each paraffin block cassette; and (iii) labels including the DataMatrix code were automatically printed on each H&E, IHC, and/or cytological slide related to the specimen. Most hardware and computers (i.e., tissue processors, scanners and stainers, etc.) of the involved institutions will be equipped with barcode readers integrated with the LIS, enabling the working team to check the samples safely during the subsequent processing steps.
As previously mentioned, for some regulatory entities [20], the recommendations advise prolonged tissue conservation after the final diagnosis, covering the whole patient’s life. The LIS automatically records a complete audit trail of each block and slide. The system relies on a modular system, progressively adding more units to its capacity without limiting the number of collectible items for short and long storage periods.
Validation
Validation is defined as a process that demonstrates that WSIs will perform as expected for their intended use and environment before using them for patient care. Regarding the validation of the entire digital network (scanners, displays and AI tools, etc.) for primary diagnosis, the recommendation for each institution is to follow the College of American Pathologists (CAP) guidelines [24], to ensure the safety and robustness of the project. The validation process must include a sample set of at least 60 cases for one application, or use case, that reflects the spectrum and complexity of specimen types. Several pathologists per center are experienced in DP and will add value to the validation process, randomly evaluating the selected WSIs previously assessed by conventional light microscopy (LM) after the washout period. Although all discordances between WSI and glass-slide diagnoses discovered during validation need to be reconciled, a 95% diagnostic concordance threshold for the same observer between LM and WSI will be the goal, according to the CAP guidelines. As far as cytopathology is concerned, widely acknowledged validating guidelines are still lacking [13]. Hence, an expert-based consensus on the non-inferiority of virtual to glass slides reviewed by dedicated cytopathologists was chosen as a reliable criterion for safely adopting the WSI technique in this field.
Discussion
In the past few decades, DP and AI have represented disruptive technologies in physicians’ ways of viewing their daily work, meeting the pressing scientific community’s demand for refined and reproducible diagnoses in the modern era of precision medicine. Despite great technological progress, the adoption of DP for routine clinical diagnostic use remains limited, and the complete transition to DP of a pathology network constitutes an organizational, functional, and technical challenge. Generally, the need for significant investment, along with pathologists’ skepticism of the adoption of new technologies has hampered comprehensive DP employment by many institutions. These considerations notwithstanding, plenty of solutions supporting the complete automatization of a pathology laboratory workflow are now available [25] and the number of departments and networks fully embracing DP for primary diagnosis keeps on increasing worldwide [26]. Since the first pioneer institutions such as the Memorial Sloan Kettering Cancer Center in the USA [27], many laboratories have followed in their footsteps, resulting in the modern broad comprehensive regional systems such as the Catalonian DigiPatICS [10], among others.
Regarding Italy, WSI-based routine diagnosis has been limited to a few isolated (but still illuminating) models, such as the frozen-section telepathology service in South Tyrol [9] and the locally connected network in Eastern Sicily [7, 28]. Thus, the digital transformation of the network of public pathology laboratories in the Italian Veneto region reported herein certainly represents a milestone for the further widespread adoption of this revolutionary technology in Italy. The realization of the present project involved a network of 12 hospitals producing more than 2.5 million slides per year, with a number that is likely to increase. Although such a process has required a large amount of public funds and resources, it will remarkably decrease operational and asset costs in the coming years. Several studies have outlined an estimated saving of more than $250,000 per institution per year after the implementation of DP [15], making the achievement of such a goal in the Veneto region likely as well.
Alongside the economic aspects, the broad deployment of DP, WSI, and AI in laboratories in Veneto will play a crucial role in facilitating the exchange of information among the involved centers. Not only will this enhance the standardization and reproducibility of pathology reports but it will also help physicians establish experienced second opinions to improve overall patients’ clinical management. Apart from the evaluation of routine biopsy and surgical specimens, it is worth mentioning the implications of knowledge and opinion-sharing in two particular fields of pathology: intraoperative consultation and cytopathology. For the former, on-site evaluation of frozen sections is supposed to quickly provide surgeons in the operating theater with the most appropriate pathological response possible. Also, especially in the transplantation setting, rendering widely reproducible diagnoses is not usually straightforward due to subtle histological changes influencing diagnostics classifications. Modern DP-based cockpits significantly help overcome such an issue [29, 30], offering the opportunity to get real-time opinions from remote, experienced colleagues. Hence, the broad network of online connected hospitals will let pathologists, particularly the youngest ones, exploit a teleconsultation service to share the most challenging cases regardless of their physical location. Together with obvious advantages in terms of clinical care, this system will provide a striking tool to help young professionals face the compelling situations of intraoperative consultation. For cytopathology, to date, cervical screening smears and other materials (e.g., thyroid fine-needle aspirations and effusion cytology) account for a noteworthy percentage of many laboratories’ routine workflows [31]. Nevertheless, the CAP still considered cytopathology as immature for diagnostic DP adoption [24]. Although soon to be released, validation guidelines for employing WSIs for primary diagnosis are still missing [13]. This fact is probably linked to the particular characteristics of cytological specimens and the lack of specifically designed validation studies. As proposed in other recent studies [32], at the time of registration of the current project, the investigators agreed to a consensus-based method to judge the feasibility and safety of WSIs in cytopathology; however, the increasing amount of data that will be progressively collected and shared by the connected hospitals of the network will perfectly suit the need for a broad multicenter study strictly following new guidelines to support the validation of WSIs for cytopathology.
Finally, the huge number of archived WSIs will create large pathology repositories for educational purposes and future technological developments. In particular, a consistent number of linked institutions gathering samples from a highly variable range of inpatient and outpatient services will help to build and expand web-based libraries of pathology fields that are often limited by the scarcity of examples constituting the cohorts (transplantation and neurosurgery, etc.) [33]. This will represent an incredible teaching opportunity for residents, students, and young pathologists to widen their knowledge. Similarly, the easily accessible massive amount of digital data produced will certainly advance the further adoption of AI-guided diagnoses, serving both as external validation datasets for already available algorithms and as the basis for developing new home-built ones.
In conclusion, this proof of concept shows which assessments were made that guided the choices relating to the tools and organizational processes in planning the DP project of the Veneto region. The Veneto DP program aims to transform 12 pathology laboratories from public hospitals producing more than 2.5 million slides per year in a coordinated network of services completely adopting WSI technology. Such an outstanding achievement has been made possible by the integration of all the health information systems of the participating institutions, along with the tireless work of dedicated pathologists, informatics, engineers, and clinicians involved in the project. The preliminary results emerging from the use of the employed LIS, devices, and AI tools firmly confirm and support the safe and effective nature of the entire project.
Data availability
All data generated or analyzed during this study are included in this published article.
Change history
23 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00428-024-03834-4
References
Zarella MD, Bowman D, Aeffner F, Farahani N, Xthona A, Absar SF, Parwani A, Bui M, Hartman DJ (2019) A practical guide to whole slide imaging: a white paper from the Digital Pathology Association. Arch Pathol Lab Med 143:222–234. https://doi.org/10.5858/arpa.2018-0343-RA
Tizhoosh HR, Pantanowitz L (2018) Artificial intelligence and digital pathology: challenges and opportunities. J Pathol Inform 9:38. https://doi.org/10.4103/jpi.jpi_53_18
N. Caldonazzi, P.C. Rizzo, A. Eccher, I. Girolami, G.N. Fanelli, A.G. Naccarato, G. Bonizzi, N. Fusco, G. d’Amati, A. Scarpa, L. Pantanowitz, S. Marletta (2023) Value of artificial intelligence in evaluating lymph node metastases., Cancers (Basel). 15. https://doi.org/10.3390/cancers15092491.
Girolami I, Pantanowitz L, Marletta S, Hermsen M, van der Laak J, Munari E, Furian L, Vistoli F, Zaza G, Cardillo M, Gesualdo L, Gambaro G, Eccher A (2022) Artificial intelligence applications for pre-implantation kidney biopsy pathology practice: a systematic review. J Nephrol 35:1801–1808. https://doi.org/10.1007/s40620-022-01327-8
Pantanowitz L, Sinard JH, Henricks WH, Fatheree LA, Carter AB, Contis L, Beckwith BA, Evans AJ, Lal A, Parwani AV (2013) Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med 137:1710–1722. https://doi.org/10.5858/arpa.2013-0093-CP
C. Eloy, J. Vale, M. Curado, A. Polónia, S. Campelos, A. Caramelo, R. Sousa, M. Sobrinho-Simões (2021). Digital pathology workflow implementation at IPATIMUP., Diagnostics (Basel, Switzerland). 11. https://doi.org/10.3390/diagnostics11112111.
F. Fraggetta, A. Caputo, R. Guglielmino, M.G. Pellegrino, G. Runza, V. L’Imperio (2021) A survival guide for the rapid transition to a fully digital workflow: the “Caltagirone example”., Diagnostics (Basel, Switzerland). 11. https://doi.org/10.3390/diagnostics11101916.
Retamero JA, Aneiros-Fernandez J, Del Moral RG (2020) Complete digital pathology for routine histopathology diagnosis in a multicenter hospital network. Arch Pathol Lab Med 144:221–228. https://doi.org/10.5858/arpa.2018-0541-OA
Girolami I, Neri S, Eccher A, Brunelli M, Hanna M, Pantanowitz L, Hanspeter E, Mazzoleni G (2022) Frozen section telepathology service: efficiency and benefits of an e-health policy in South Tyrol. Digit. Heal. 8:20552076221116776. https://doi.org/10.1177/20552076221116776
J. Temprana-Salvador, P. López-García, J. Castellví Vives, L. de Haro, E. Ballesta, M. Rojas Abusleme, M. Arrufat, F. Marques, J.R. Casas, C. Gallego, L. Pons, J.L. Mate, P.L. Fernández, E. López-Bonet, R. Bosch, S. Martínez, S. Ramón Y Cajal, X. Matias-Guiu (2022) DigiPatICS: digital pathology transformation of the Catalan Health Institute Network of 8 hospitals-planification, implementation, and preliminary results. Diagnostics (Basel, Switzerland). 12. https://doi.org/10.3390/diagnostics12040852.
Rizzo PC, Caputo A, Maddalena E, Caldonazzi N, Girolami I, Dei Tos AP, Scarpa A, Sbaraglia M, Brunelli M, Gobbo S, Marletta S, Pantanowitz L, Della Mea V, Eccher A (2023) Digital pathology world tour. Digit. Heal. 9:20552076231194550. https://doi.org/10.1177/20552076231194551
Rizzo PC, Girolami I, Marletta S, Pantanowitz L, Antonini P, Brunelli M, Santonicco N, Vacca P, Tumino N, Moretta L, Parwani A, Satturwar S, Eccher A, Munari E (2022) Technical and diagnostic issues in whole slide imaging published validation studies. Front. Oncol. 12:918580. https://doi.org/10.3389/fonc.2022.918580
Antonini P, Santonicco N, Pantanowitz L, Girolami I, Rizzo PC, Brunelli M, Bellevicine C, Vigliar E, Negri G, Troncone G, Fadda G, Parwani A, Marletta S, Eccher A (2023) Relevance of the College of American Pathologists guideline for validating whole slide imaging for diagnostic purposes to cytopathology. Cytopathology 34:5–14. https://doi.org/10.1111/cyt.13178
Regione del Veneto, Azienda Zero. https://www.azero.veneto.it/. Accessed 25 Sept 2023
Patel A, Balis UGJ, Cheng J, Li Z, Lujan G, McClintock DS, Pantanowitz L, Parwani A (2021) Contemporary whole slide imaging devices and their applications within the modern pathology department: a selected Hardware Review. J Pathol Inform. 12:50. https://doi.org/10.4103/jpi.jpi_66_21
Marletta S, Treanor D, Eccher A, Pantanowitz L (2021) Whole-slide imaging in cytopathology: state of the art and future directions. Diagnostic Histopathol. https://doi.org/10.1016/j.mpdhp.2021.08.001
Pantanowitz L, Harrington S (2021) Experience reviewing digital pap tests using a gallery of images. J Pathol Inform 12:7. https://doi.org/10.4103/jpi.jpi_96_20
Abel JT, Ouillette P, Williams CL, Blau J, Cheng J, Yao K, Lee WY, Cornish TC, Balis UGJ, McClintock DS (2020) Display characteristics and their impact on digital pathology: a current review of pathologists’ future “microscope.” J. Pathol. Inform. 11:23. https://doi.org/10.4103/jpi.jpi_38_20
Tesi, PathoxWeb 2.0.0 User Manual, (2023) 1–250.
Kapila SN, Boaz K, Natarajan S (2016) The post-analytical phase of histopathology practice: storage, retention and use of human tissue specimens. Int J Appl Basic Med Res 6:3–7. https://doi.org/10.4103/2229-516X.173982
Aiforia releases another CE-IVD marked clinical AI Model — expanding its diagnostic pathology offering to prostate cancer, 27th May. (2022). https://www.aiforia.com/blog/ce-ivd-prostate-cancer-gleason (accessed December 27, 2023).
Aiforia expands its portfolio of CE-IVD marked clinical pathology solutions with new AI model for breast cancer diagnostics, 20th April. (2022). https://www.aiforia.com/blog/ce-ivd-ai-model-er (accessed December 5, 2023).
Aiforia achieves CE-IVD mark for clinical PD-L1 AI model in lung cancer diagnostics, 16th Novemb. (2021). https://www.aiforia.com/blog/ce-ivd-ai-model-pd-l1 (accessed December 5, 2021).
Evans AJ, Brown RW, Bui MM, Chlipala EA, Lacchetti C, Milner DA, Pantanowitz L, Parwani AV, Reid K, Riben MW, Reuter VE, Stephens L, Stewart RL, Thomas NE (2022) Validating whole slide imaging systems for diagnostic purposes in pathology. Arch Pathol Lab Med 146:440–450. https://doi.org/10.5858/arpa.2020-0723-CP
E. Munari, A. Scarpa, L. Cima, M. Pozzi, F. Pagni, F. Vasuri, S. Marletta, A.P. Dei Tos, A. Eccher (2023) Cutting-edge technology and automation in the pathology laboratory. Virchows Arch. https://doi.org/10.1007/s00428-023-03637-z.
Schwen LO, Kiehl T-R, Carvalho R, Zerbe N, Homeyer A (2023) Digitization of pathology labs: a review of lessons learned. Lab Invest 103:100244. https://doi.org/10.1016/j.labinv.2023.100244
Ardon O, Klein E, Manzo A, Corsale L, England C, Mazzella A, Geneslaw L, Philip J, Ntiamoah P, Wright J, Sirintrapun SJ, Lin O, Elenitoba-Johnson K, Reuter VE, Hameed MR, Hanna MG (2023) Digital pathology operations at a tertiary cancer center: infrastructure requirements and operational cost. J Pathol Inform 14:100318. https://doi.org/10.1016/j.jpi.2023.100318
Fraggetta F, Garozzo S, Zannoni GF, Pantanowitz L, Rossi ED (2017) Routine digital pathology workflow: the Catania experience. J Pathol Inform. 8:51. https://doi.org/10.4103/jpi.jpi_58_17
Brunelli M, Beccari S, Colombari R, Gobbo S, Giobelli L, Pellegrini A, Chilosi M, Lunardi M, Martignoni G, Scarpa A, Eccher A (2014) iPathology cockpit diagnostic station: validation according to College of American Pathologists Pathology and Laboratory Quality Center recommendation at the Hospital Trust and University of Verona. Diagn Pathol 9(Suppl 1):S12. https://doi.org/10.1186/1746-1596-9-S1-S12
Marletta S, Pantanowitz L, Malvi D, Novelli L, Mescoli C, Cardillo M, D’Errico A, Girolami I, Eccher A (2022) Validation of portable tablets for transplant pathology diagnosis according to the College of American Pathologists Guidelines. Acad Pathol 9:100047. https://doi.org/10.1016/j.acpath.2022.100047
Santonicco N, Marletta S, Pantanowitz L, Fadda G, Troncone G, Brunelli M, Ghimenton C, Antonini P, Paolino G, Girolami I, Eccher A (2022) Impact of mobile devices on cancer diagnosis in cytology. Diagn Cytopathol 50:34–45. https://doi.org/10.1002/dc.24890
Marletta S, Salatiello M, Pantanowitz L, Bellevicine C, Bongiovanni M, Bonoldi E, De Rezende G, Fadda G, Incardona P, Munari E, Pagni F, Rossi ED, Tallini G, Troncone G, Ugolini C, Vigliar E, Eccher A (2023) Delphi expert consensus for whole slide imaging in thyroid cytopathology. Cytopathology 34:581–589. https://doi.org/10.1111/cyt.13279
Neri F, Eccher A, Rigotti P, Girolami I, Zaza G, Gambaro G, Mastrosimini M, Bencini G, Di Bella C, Mescoli C, Boschiero L, Marletta S, Tos PAD, Furian L (2021) Advantages of using a web-based digital platform for kidney preimplantation biopsies. J Pathol Inform 12:41. https://doi.org/10.4103/jpi.jpi_23_21
Acknowledgements
None.
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement. This study was funded by the “European Union—NextGenerationEU through the Italian Ministry of University and Research under PNRR—M4C2-I1.3 Project PE_00000019 “HEAL ITALIA” CUP: B33C22001030006.”
Author information
Authors and Affiliations
Contributions
Conceptualization: A.E. and A.P.D.T.; methodology: A.E., S.M., and A.G.; formal analysis and investigation: A.E., S.M., and A.G.; writing—original draft preparation: A.E., S.M., and A.G.; writing—review and editing: M.S., M.R., G.G., and A.S; supervision: A.E., A.S., and A.P.D.T.
Corresponding author
Ethics declarations
Ethical approval
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eccher, A., Marletta, S., Sbaraglia, M. et al. Digital pathology structure and deployment in Veneto: a proof-of-concept study. Virchows Arch (2024). https://doi.org/10.1007/s00428-024-03823-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00428-024-03823-7