Abstract
Somatic and biallelic DICER1 mutations are reported in subsets of thyroid tumors, supporting the role of this gene in thyroid tumor development. As recent studies have brought attention to macrofollicular patterns, atrophic changes, and papillary structures as being associated with DICER1 mutations, we sought to explore these observations in a bi-institutional cohort. A total of 61 thyroid lesions (54 tumors and 7 cases of thyroid follicular nodular disease; TFND), including 26 DICER1 mutated and 35 DICER1 wildtype controls were subjected to histological re-investigation and clinical follow-up. DICER1-mutated lesions showed a statistically significant association with younger age at surgery (29.2 ± 12.5 versus 51.3 ± 18.8, p = 0.0001), a predominant macrofollicular growth pattern (20/26 mutated cases versus 18/35 wildtype; p = 0.01) and atrophic changes (20/26 mutated cases versus 2/35 wildtype; p = 0.0001). Similar results were obtained when excluding TFND cases. We also present clinical and histological triaging criteria for DICER1 sequencing of thyroid lesions, which led to the identification of DICER1 variants in 16 out of 26 cases (62%) when followed. Among these, 3 out of 12 cases with available data were found to carry a constitutional DICER1 mutation. This observation suggests that the majority of DICER1 mutations are somatic—however implies that sequencing of constitutional tissues could be clinically motivated. We conclude that DICER1 mutations are amassed in younger patients with macrofollicular-patterned tumors and, most strikingly, atrophic changes. Given the rate of constitutional involvement, our findings could be of clinical value, allowing the pathologist to triage cases for genetic testing based on histological findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hereditary cancer syndromes account for approximately 10% of all cancers [1]. The proportion of patients with thyroid tumors exhibiting a monogenic, syndromic form of the disease is very low when considering tumors from follicular-derived cells [2]. The most established entities in this context are the McCune-Albright and PTEN syndromes, where a certain proportion of patients develops follicular thyroid tumors [3,4,5,6].
The discovery of two separate syndromes caused by mutations in genes controlling the maturation of micro-RNA (DICER1 and DGCR8) has been particularly interesting from a thyroid point of view, as these patients develop thyroid follicular nodular disease (TFND) and thyroid tumors [7, 8]. The DICER1 syndrome is an autosomal dominant disorder that places carriers at risk of developing multiple tumors and tumor-like conditions in the thyroid gland, lungs, gonads, and soft tissues [9]. The prevalence of this syndrome is relatively unknown but is believed to increase as general knowledge grows, leading to an intensification of clinical screening efforts [10]. The majority of patients with this syndrome carries an inactivating mutation in the DICER1 gene, typically a prematurely truncated variant upstream of the functionally important RNAse III domain [11, 12]. However, missense mutations, in-frame deletions, and intronic variants affecting splicing have also been demonstrated [13,14,15]. Tumors in the context of this syndrome often exhibit a two-hit inactivation, where a somatic mutation is recruited on the second allele—an alteration frequently located in the functionally important domain that controls the processing of micro-RNA [16, 17]. For this reason, the gene is considered to be a tumor suppressor, which has also been demonstrated functionally in genetically engineered mice [18]. In contrast to constitutional mutations, somatic DICER1 mutations typically occur at specific positions that are important for metal-ion binding such as the hotspot amino acids 1705, 1709, 1809, 1810, and 1813 [17].
Concerning sporadic thyroid tumors, it is known that mutations in this gene occur in a subset of TFND, follicular thyroid adenoma (FTA), non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), follicular thyroid carcinomas (FTC), and the invasive encapsulated follicular variant of papillary thyroid cancer (IEFVPTC) [19,20,21,22,23]. Mutations also manifest in a subset of high-grade thyroid tumors in pediatric patients, indicating a broad spectrum [24]. Recent work demonstrates a frequency of less than 5% for these mutations in various follicle cell-derived tumors [25]. Most of the DICER1 mutations are somatic and typically do not occur concurrently with other driver mutations. This suggests that the gene has the potential to independently drive tumor development.
Interestingly, specific histologic attributes have been associated with these mutations. For instance, macrofollicular structures, “abortive areas,” and focal papillary growth have been associated with the presence of a DICER1 mutation [26, 27].
In this study, we aimed to delineate these phenomena in a bi-institutional cohort of thyroid lesions with DICER1 mutation, to potentially confirm the hypothesis that the mutation gives rise to specific histological attributes observable under the microscope. Furthermore, by employing a set of clinical and histologic inclusion criteria in clinical practice, we have attempted to evaluate how these factors might contribute to an increased proportion of mutation-positive findings in the clinical setting.
Material and methods
Detailed case description
A total of 61 thyroid lesions with available DICER1 genotype (54 tumors and 7 TFND) were studied, including 26 DICER1-mutated cases and 35 wildtype controls. The summarized cohort information is shown in Table 1. Cases were collected from two independent institutions: 26 lesions from 26 patients diagnosed at the Karolinska University Hospital, Stockholm, Sweden, during 2021–2023 (Karolinska cohort), and 35 lesions from 33 patients diagnosed at Emory University Hospital, Atlanta, and Children’s Healthcare of Atlanta, both in Georgia, USA, collected during 2018–2023 (Emory/CHOA cohort). All Karolinska cases are previously unpublished, as are the majority of the Emory/CHOA cohort cases [27]. Ethical permission was granted through the Swedish Ethical Review Authority (approvals #2015.959–31 and # 2020–00281) and the Institutional Review Board (IRB#00004304), that includes both Emory University and Children’s Healthcare of Atlanta.
At Karolinska, routine preoperative assessments of thyroid nodules do not typically involve next-generation sequencing (NGS) or direct sequencing of thyroid-related genes, except in rare cases. Hence, thyroid nodules from Karolinska that underwent DICER1 sequencing by NGS were chosen based on clinical and histological screening, requiring the fulfillment of at least one among several specific criteria, and thereafter sequenced using DNA extracted from postoperative, formalin-fixated paraffin-embedded (FFPE) specimen (Fig. 1). The inclusion criteria for DICER1 sequencing at Karolinska were (1) TFND in patients < 25 years and with typical histology (multiple adenomatoid nodules with papillae), (2) histological diagnosis of a macrofollicular thyroid tumor (FTA, FTC, papillary thyroid carcinoma; PTC) irrespectively of age, (3) conventional FTC in patients < 25 years, and (4) differentiated high-grade thyroid carcinoma (DHGTC) or poorly differentiated thyroid carcinoma (PDTC) in patients < 50 years. Meaning, all cases from Karolinska in this study have fulfilled one of the abovementioned criteria, irrespective of whether they harbored a DICER1 gene mutation or not.
For the Emory/CHOA cohort, the respective pathology archives were queried for indeterminate cytology cases that underwent Thyroseq v3 testing and found to have a DICER1 mutation. Corresponding surgical resection samples, when available, were collected and reviewed. In addition to the DICER1-mutated cases, a separate group of resected DICER1 wildtype, RAS-like mutant thyroid lesions were selected to serve as control cases.
Baseline clinical and histopathological data were gathered for the entire study cohort by reviewing patient charts and pathology reports. Additionally, a meticulous histological review was conducted, focusing on specific histological attributes. Specifically, atrophic changes, previously referred to as “abortive changes” [26], were characterized by the presence of multiple areas in which the tumor tissue appeared atrophic and palely stained compared to the surrounding tissue, with ghost-cell-like areas exhibiting clear cut demarcation compared to the unaffected, adjacent tumor tissues. Usually, these areas exhibited thickening of the interstitial stroma and a reduction of viable cells. These changes were frequently observed in tumor regions adjacent to the capsule. It is important to note that our definition of atrophic changes excludes features such as associated fibrosis, inflammation, and cellular debris. This exclusion serves to safely eliminate potential artifacts stemming from post-fine needle aspiration biopsy.
DICER1 sequencing of thyroid lesions
For Karolinska cases, DNA was extracted from FFPE and sequenced using the Oncomine Childhood Cancer NGS platform (Thermo-Fisher, Waltham, MA, USA) used in clinical routine at the Department of Pathology and Cancer Diagnostics. This platform includes the entire coding sequence and intron–exon boundaries of DICER1, in addition to a number of other cancer-related genes and fusions, e.g., BRAF, NRAS, HRAS, and KRAS to name a few. The report obtained includes the DICER1 genotype and allele frequency of each mutation found. All samples were confirmed as containing high tumor purity via histological investigation of a representative section from the DNA extraction process.
For the Emory/CHOA, the indeterminate cases on cytology underwent Thyroseq v3 testing as described previously [28], which identified a DICER1 or a RAS-like alteration (e.g., RAS, EIF1AX).
Constitutional DICER1 mutation detection
A subset of Karolinska cases with identified DICER1 mutation in the thyroid lesion underwent scrutiny for constitutional involvement through the Clinical Genetics Department, utilizing an NGS platform employed in routine clinical practice.
Statistical analyses
Fisher’s exact test and Mann–Whitney-U test were used to compare clinicopathological features between DICER1-mutated and wildtype cases, and a p-value < 0.05 was considered statistically significant. Statistical computations were performed using GraphPad Prism 10.1.1 (GraphPad Software, San Diego, California USA).
Results
DICER1 genotype and correlations to clinical and histological variables
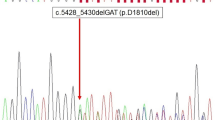
Out of the 61 thyroid lesions (54 tumors and 7 cases of TFND), 26 lesions harbored DICER1 mutations and the remaining 35 were wildtype. Genetic data, clinical, and histological features for all DICER1-mutated lesions are detailed in Table 2. Totally, 25 of the 26 DICER1-mutated thyroid lesions exhibited a hotspot mutation, which was shown to be somatic in all 14 for which constitutional DNA was sequenced. Another thyroid lesion exhibited a nonsense mutation that was shown to be constitutional.
In brief, the 26 DICER1-mutated thyroid lesions include 3 TFND cases, 8 benign/low-risk lesions (7 FTA and 1 NIFTP), 10 well-differentiated thyroid carcinomas (10 FTC), and 5 high-grade thyroid carcinomas (3 DHGTC and 2 PDTC). The 35 DICER1 wildtype thyroid lesions consist of 4 TFND cases, 13 benign/low-risk lesions (8 FTA, 4 follicular thyroid tumors of uncertain malignant potential; FT-UMP, 1 NIFTP), 16 well-differentiated thyroid carcinomas (13 FTC, 3 oncocytic thyroid carcinoma; OTC), and 2 high-grade thyroid carcinomas (both DHGTC).
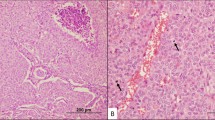
The results are summarized in Fig. 2. DICER1-mutated cases displayed a younger age at surgery (29.2 ± 12.5 vs. 51.3 ± 18.8, p ≤ 0.0001), a predominant macrofollicular growth pattern (20/26 mutated cases vs. 18/35 wildtype; p = 0.01) and atrophic changes (20/26 mutated cases vs. 2/35 wildtype; p ≤ 0.0001) (Table 3; Fig. 3). Papillary formations were not statistically significantly overrepresented among DICER1-mutated lesions but were a recurrent feature of TFND cases with DICER1 mutations (Table 3; Fig. 4). Notably, atrophic changes were highly sensitive and specific for the detection of DICER1 mutations. In the thyroid tumors studied, atrophic changes were noted in 20 out of 26 DICER1-mutated cases (77%), compared to 2 out of 35 DICER1 wild-type cases (5%). Thus, the sensitivity and specificity of atrophic changes as a harbinger of DICER1 mutations would reach 77% and 95% respectively. Atrophic changes were observed both in cases with somatic as well as constitutional variants, as illustrated in Fig. 3, and there was no apparent association with mutational status in this regard.
A Atrophic changes in case KI 2, a minimally invasive follicular thyroid carcinoma (MIFTC) with macrofollicular architecture in a patient with constitutional DICER1 mutation. Widespread areas with palely stained tumor cells (termed “atrophic changes”) were noted adjacent to the non-affected tumor cells (asterisk). The absence of cellular debris and inflammation argues against necrosis, and there are no apoptotic bodies. Magnification × 100. B High-magnification (× 400) photomicrograph of the same case, highlighting the atrophic changes. These features are sensitive and highly specific for DICER1 mutations in follicular-patterned thyroid tumors. C Case KI 8 at × 40 magnification, a macrofollicular variant follicular thyroid adenoma with somatic DICER1 mutation. Note the brisk transition between atrophic changes (arrowheads) and unaffected tumor tissue. D High-power magnification of the same case
Main histological attributes of KI 1 case, a thyroid follicular nodular disease (TFND) in a young female patient with germline DICER1 mutation. A Macroscopic image. Note the heterogeneous appearance with multiple adenomatoid-like nodules visible at grossing. B Hematoxylin and eosin (H&E) staining of the same case, displaying colloid nodules mixed with microfollicular-patterned adenomatous areas. C–D Areas with prominent papillary formations are evident. Nuclear atypia is absent
Constitutional DICER1 sequencing outcome
In the Karolinska cohort, DICER1 mutations were found in 16 out of 26 cases (62%) fulfilling the inclusion criteria. In cases with available germline data (n = 12), 3 cases (25%) were found to carry constitutional DICER1 nonsense mutations: in one patient with TFND and two patients with minimally invasive FTC (MIFTC) with macrofollicular growth. These patients were females aged 13, 15, and 29 at surgery. Two of these patients exhibited a family history of TFND, and the grandmother of an additional patient was previously diagnosed with thyroid carcinoma. In the remaining 9 cases, no constitutional DICER1 mutation was identified.
In the Emory/CHOA cohort, two patients with DICER1-mutated thyroid lesions identified on Thyroseq v3 underwent constitutional DNA testing. One female patient aged 15 with a PDTC and a separate FTA exhibited a constitutional DICER1 missense variant of uncertain significance (VUS) (Table 2). The second patient tested, aged 17 and female, had a MIFTC on surgery. This patient did not have a constitutional DICER1 alteration and no significant family history.
Overall, based on these results, we suggest an algorithm, summarized in Fig. 5, that could be used in centers that routinely do not perform universal genetic testing in thyroid nodules, to better predict DICER1 mutations using various clinical and histological criteria.
DICER1 screening in thyroid lesions using clinical and histological harbingers: a suggested algorithm for centers that do not perform universal genetic testing in thyroid nodules. *Data from the current study with a limited number of Karolinska patients undergoing germline sequencing. PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; OTC, oncocytic thyroid carcinoma; DHGTC, differentiated high-grade thyroid carcinoma; PDTC, poorly differentiated thyroid carcinoma. Created using BioRender.com
Discussion
Characterized for the first time in 2009, the DICER1 syndrome is a multi-tumor condition caused by germline pathogenetic variants in the DICER1 gene and associated with different neoplastic lesions affecting the lungs, thyroid gland, ovaries, and kidney [7]. For the syndrome to occur, typically, a loss-of-function variant, resulting in the inactivation of the affected DICER1 allele, and a second “hotspot” pathogenetic mutation affecting the RNase IIIb domain of DICER1 are required [12].
Patients with DICER1 syndrome, typically in pediatric or adolescent age, face an increased risk of developing a variety of thyroid lesions, spanning from TFND to malignant thyroid tumors [29,30,31]. Even though this syndrome is important to recognize, it should be stressed that somatic DICER1 aberrations in thyroid nodules are likely to be more prevalent than germline mutations [17]. Given the rather widespread occurrence of these somatic mutations in thyroid nodules, there is a significant clinical need to ascertain the clinical relevance and extent of these alterations, as well as whether the finding of these alterations would require germline testing to rule out syndromic disease [19, 21, 32, 33]. Moreover, as the detection rate of DICER1 mutations in thyroid tumors will rise as more pathology centers rely on next-generation sequencing, identifying potential genotype–phenotype correlations is crucial, especially for centers that require specific conditions for case submission for sequencing.
In this study, we gathered molecular, histopathological, and clinical data from thyroid tumors at two distinct institutions (European and North American) to examine whether our initial observations regarding a genotype–phenotype correlation remain consistent in larger cohorts obtained from diverse centers. Our investigation affirms that specific histological features, such as macrofollicular growth and atrophic changes distinctly correlate with the presence of DICER1 mutation in follicular-patterned thyroid tumors, corroborating the previously assumed association between macrofollicular variants of follicular thyroid tumors and inactivating somatic DICER1 mutations reported in previous studies [26, 27, 34, 35]
Interestingly, the identification of atrophic areas seems to be strongly associated with this genetic aberrancy, as only 2 out of 35 DICER1 wildtype cases with RAS mutations exhibited such areas. It is not known if atrophic changes are unique to follicular cell-derived thyroid tumors or if they are also visualized in unrelated thyroid tumors with DICER1 mutations, for example, thyroblastoma [36, 37]. Upon reviewing the current literature, we did not observe such an association. However, future research investigating the potential role of atrophic changes in this tumor type would indeed be of interest in order to increase our understanding of this phenomenon.
Moreover, while atrophic changes have not yet been described on the cytological level, there are reports suggesting that macrofollicular structures and papillary excrescences may also be detected in fine needle aspirates of DICER1-mutated thyroid nodules [38]. Further studies in cytological preparations could possibly help delineate important clues already at the preoperative level. The specific mechanism underlying the atrophic changes is not known, but one might speculate that the aberrant micro-RNA expression patterns conferred by the DICER1 mutations potentially instigate an atrophic state. Indeed, several micro-RNAs have been associated with thyrocyte maturation and differentiation [39]. Moreover, DICER1 deficiency in the retinal pigment epithelium has been associated with oxidative stress and geographic atrophy, and DICER1 knock-out mice develop prostate atrophy [40, 41].
The DICER1-mutated cohort from Karolinska was assembled via NGS analysis, specifically targeting cases that exhibited specific epidemiologic and/or histologic criteria. By screening only those lesions meeting specific criteria, the percentage of positive cases—indicating patients with the aforementioned mutation—comprises > 50% of the total material. This should be compared to observations made in a large, unselected material of > 400 TFND and follicular-patterned tumors in which the DICER1 hotspot mutational frequency was approximately 3% [25]. This underscores our demonstration that employing specific criteria can substantially elevate the proportion of patients with detected DICER1 mutation in clinical practice.
In the present study, not all patients underwent analysis for mutations in constitutional tissue. Additionally, prior research indicates that most of these mutations are somatic [19, 20]. The critical question, therefore, revolves around the significance of detecting such a genetic aberration in clinical routine. Presumably, this approach allows pathologists to guide cases to surgeons for further clinical genetic management. Although the majority of patients may test negative for constitutional mutations, identifying individuals with syndromic forms of the disease holds substantial clinical value, justifying meticulous genetic mapping. Nevertheless, firm conclusions in this regard necessitate larger studies with available constitutional data.
To summarize, based on morphological and histological characteristics, we suggest an algorithm that could be used to predict DICER1 mutations in follicular-patterned thyroid tumors a priori. In particular, specific features such as macrofollicular growth and atrophic changes appear to be strongly coupled to DICER1 mutations in follicular-pattern thyroid tumors. Potentially, these histological attributes, together with young age, may display a specific and unique combination that could be of clinical interest, allowing pathologists to categorize cases for genetic testing based on histological findings.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Rahman N (2014) Realizing the promise of cancer predisposition genes. Nature 505:302–308. https://doi.org/10.1038/nature12981
Nosé V, Gill A, Teijeiro JMC et al (2022) Overview of the 2022 WHO classification of familial endocrine tumor syndromes. Endocr Pathol 33:197–227. https://doi.org/10.1007/s12022-022-09705-5
Albright F, Butler AM, Hampton AO, Smith P (1937) Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females: report of five cases. N Engl J Med 216:727–746. https://doi.org/10.1056/NEJM193704292161701
Danon M, Crawford JD (1987) The McCune-Albright syndrome. In: Frick P, Harnack G-A, Kochsiek K et al (eds) Ergebnisse der Inneren Medizin und Kinderheilkunde/Advances in Internal Medicine and Pediatrics. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 81–115
Dumitrescu CE, Collins MT (2008) McCune-Albright syndrome. Orphanet J Rare Dis 3:12. https://doi.org/10.1186/1750-1172-3-12
Yehia L, Ngeow J, Eng C (2019) PTEN-opathies: from biological insights to evidence-based precision medicine. J Clin Investig 129:452–464. https://doi.org/10.1172/JCI121277
Hill DA, Ivanovich J, Priest JR et al (2009) DICER1 mutations in familial pleuropulmonary blastoma. Science 325:965. https://doi.org/10.1126/science.1174334
Rivera B, Nadaf J, Fahiminiya S et al (2020) DGCR8 microprocessor defect characterizes familial multinodular goiter with schwannomatosis. J Clin Invest 130:1479–1490. https://doi.org/10.1172/JCI130206
Kommoss FKF, Chong A-S, Chong A-L et al (2023) Genomic characterization of DICER1-associated neoplasms uncovers molecular classes. Nat Commun 14:1677. https://doi.org/10.1038/s41467-023-37092-w
Kim J, Field A, Schultz KAP et al (2017) The prevalence of DICER1 pathogenic variation in population databases. Intl Journal of Cancer 141:2030–2036. https://doi.org/10.1002/ijc.30907
Heravi-Moussavi A, Anglesio MS, Cheng S-WG et al (2012) Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med 366:234–242. https://doi.org/10.1056/NEJMoa1102903
Foulkes WD, Priest JR, Duchaine TF (2014) DICER1: mutations, microRNAs and mechanisms. Nat Rev Cancer 14:662–672. https://doi.org/10.1038/nrc3802
Rio Frio T, Bahubeshi A, Kanellopoulou C et al (2011) DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA 305:68–77. https://doi.org/10.1001/jama.2010.1910
Apellaniz-Ruiz M, Sabbaghian N, Chong A-L et al (2023) Reclassification of two germline DICER1 splicing variants leads to DICER1 syndrome diagnosis. Fam Cancer 22:487–493. https://doi.org/10.1007/s10689-023-00336-1
Apellaniz-Ruiz M, de Kock L, Sabbaghian N et al (2018) Familial multinodular goiter and Sertoli-Leydig cell tumors associated with a large intragenic in-frame DICER1 deletion. Eur J Endocrinol 178:K11–K19. https://doi.org/10.1530/EJE-17-0904
Wu MK, Sabbaghian N, Xu B et al (2013) Biallelic DICER1 mutations occur in Wilms tumours. J Pathol 230:154–164. https://doi.org/10.1002/path.4196
Juhlin CC (2023) On the chopping block: overview of DICER1 mutations in endocrine and neuroendocrine neoplasms. Surg Pathol Clin 16:107–118. https://doi.org/10.1016/j.path.2022.09.010
Wang Y, Chen SY, Ta M et al (2023) Biallelic Dicer1 mutations in the gynecologic tract of mice drive lineage-specific development of DICER1 syndrome-associated cancer. Cancer Res 83:3517–3528. https://doi.org/10.1158/0008-5472.CAN-22-3620
Onder S, Mete O, Yilmaz I et al (2022) DICER1 mutations occur in more than one-third of follicular-patterned pediatric papillary thyroid carcinomas and correlate with a low-risk disease and female gender predilection. Endocr Pathol 33:437–445. https://doi.org/10.1007/s12022-022-09736-y
Ricarte-Filho JC, Casado-Medrano V, Reichenberger E et al (2023) DICER1 RNase IIIb domain mutations trigger widespread miRNA dysregulation and MAPK activation in pediatric thyroid cancer. Front Endocrinol 14:1083382. https://doi.org/10.3389/fendo.2023.1083382
Ghossein CA, Dogan S, Farhat N et al (2022) Expanding the spectrum of thyroid carcinoma with somatic DICER1 mutation: a survey of 829 thyroid carcinomas using MSK-IMPACT next-generation sequencing platform. Virchows Arch 480:293–302. https://doi.org/10.1007/s00428-021-03212-4
Chong A-S, Nikiforov YE, Condello V et al (2021) Prevalence and spectrum of DICER1 mutations in adult-onset thyroid nodules with indeterminate cytology. J Clin Endocrinol Metab 106:e968–e977. https://doi.org/10.1210/clinem/dgab025
Poma AM, Condello V, Denaro M et al (2019) DICER1 somatic mutations strongly impair miRNA processing even in benign thyroid lesions. Oncotarget 10:1785–1797. https://doi.org/10.18632/oncotarget.26639
Chernock RD, Rivera B, Borrelli N et al (2020) Poorly differentiated thyroid carcinoma of childhood and adolescence: a distinct entity characterized by DICER1 mutations. Mod Pathol 33:1264–1274. https://doi.org/10.1038/s41379-020-0458-7
Condello V, Poma AM, Macerola E et al (2024) Prevalence, molecular landscape and clinical impact of DICER1 and DGCR8 mutated follicular-patterned thyroid nodules. J Clin Endocrinol Metab dgae034. https://doi.org/10.1210/clinem/dgae034
Juhlin CC, Stenman A, Zedenius J (2021) Macrofollicular variant follicular thyroid tumors are DICER1 mutated and exhibit distinct histological features. Histopathology 79:661–666. https://doi.org/10.1111/his.14416
Lengyel K, Lubin DJ, Hsiao W-Y et al (2024) Comprehensive evaluation of cytomorphologic, histologic, and molecular features of DICER1-altered thyroid lesions on FNA: a multipractice experience. Cancer Cytopathol. https://doi.org/10.1002/cncy.22805
Steward DL, Carty SE, Sippel RS et al (2019) Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: a prospective blinded multicenter study. JAMA Oncol 5:204–212. https://doi.org/10.1001/jamaoncol.2018.4616
Oliver-Petit I, Bertozzi A, Grunenwald S et al (2019) Multinodular goitre is a gateway for molecular testing of DICER1 syndrome. Clin Endocrinol 91:669–675. https://doi.org/10.1111/cen.14074
Kock L, Wu MK, Foulkes WD (2019) Ten years of DICER1 mutations: provenance, distribution, and associated phenotypes. Hum Mutat 40:1939–1953. https://doi.org/10.1002/humu.23877
Rutter MM, Jha P, Schultz KAP et al (2016) DICER1 mutations and differentiated thyroid carcinoma: evidence of a direct association. J Clin Endocrinol Metab 101:1–5. https://doi.org/10.1210/jc.2015-2169
Minna E, Devecchi A, Pistore F et al (2023) Genomic and transcriptomic analyses of thyroid cancers identify DICER1 somatic mutations in adult follicular-patterned RAS-like tumors. Front Endocrinol (Lausanne) 14:1267499. https://doi.org/10.3389/fendo.2023.1267499
Bae J-S, Jung S-H, Hirokawa M et al (2021) High prevalence of DICER1 mutations and low frequency of gene fusions in pediatric follicular-patterned tumors of the thyroid. Endocr Pathol 32:336–346. https://doi.org/10.1007/s12022-021-09688-9
Bongiovanni M, Sykiotis GP, La Rosa S et al (2020) Macrofollicular variant of follicular thyroid carcinoma: a rare underappreciated pitfall in the diagnosis of thyroid carcinoma. Thyroid 30:72–80. https://doi.org/10.1089/thy.2018.0607
Hellgren LS, Hysek M, Jatta K et al (2021) Macrofollicular variant of follicular thyroid carcinoma (MV-FTC) with a somatic DICER1 gene mutation: case report and review of the literature. Head and Neck Pathol 15:668–675. https://doi.org/10.1007/s12105-020-01208-1
Agaimy A, Witkowski L, Stoehr R et al (2020) Malignant teratoid tumor of the thyroid gland: an aggressive primitive multiphenotypic malignancy showing organotypical elements and frequent DICER1 alterations-is the term “thyroblastoma” more appropriate? Virchows Arch 477:787–798. https://doi.org/10.1007/s00428-020-02853-1
Rooper LM (2023) From malignant thyroid teratoma to thyroblastoma: evolution of a newly-recognized DICER1 -associated malignancy. Adv Anat Pathol 30:136–145. https://doi.org/10.1097/PAP.0000000000000364
Lee SH, Vadlamudi C, Zhao Q et al (2022) An institutional experience with DICER1 mutated thyroid nodules-evaluating the cytomorphology and molecular phenotype. J Am Soc Cytopathol 11:335–344. https://doi.org/10.1016/j.jasc.2022.07.002
Undeutsch H, Löf C, Pakarinen P et al (2015) Thyrocyte-specific Dicer1 deficiency alters thyroid follicular organization and prevents goiter development. Endocrinology 156:1590–1601. https://doi.org/10.1210/en.2014-1767
Kaarniranta K, Pawlowska E, Szczepanska J, Blasiak J (2020) DICER1 in the pathogenesis of age-related macular degeneration (AMD) - Alu RNA accumulation versus miRNA dysregulation. Aging Dis 11:851–862. https://doi.org/10.14336/AD.2019.0809
Zhang L, Zhang B, Valdez JM et al (2010) Dicer ablation impairs prostate stem cell activity and causes prostate atrophy. Stem Cells 28:1260–1269. https://doi.org/10.1002/stem.455
Funding
Open access funding provided by Karolinska Institute. This work was supported by The Swedish Cancer Society (CAN 2020/426).
Author information
Authors and Affiliations
Contributions
K.V. and C.C.J. designed the study. J.W.R., A.S., K.V., and C.C.J. collected clinical and histological information. V.C., A.S., C.L., K.V., and C.C.J. analyzed the data. V.C. and C.C.J. wrote the paper with inputs from all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study was approved by the Swedish Ethical Review Authority (EPN #2015.959–31 and # 2020–00281) and the Institutional Review Board (IRB #00004304).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Condello, V., Roberts, J.W., Stenman, A. et al. Atrophic changes in thyroid tumors are strong indicators of underlying DICER1 mutations: a bi-institutional genotype–phenotype correlation study. Virchows Arch (2024). https://doi.org/10.1007/s00428-024-03802-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00428-024-03802-y