Abstract
We present a thorough review of the literature on Riedel thyroiditis (RT) with emphasis on aetiology, diagnosis and management, using the PubMed, Sinomed, and China National Knowledge Infrastructure databases. Although the exact aetiology of RT remains obscure, the histopathological features are consistent with a localized form of IgG4-related systemic disease (IgG4-RSD). Nevertheless, IgG4-RSD as a systemic fibroinflammatory disorder per se rarely affects the thyroid in the context of multiorgan manifestations. The initial diagnosis of RT is based on clinical history and imaging, but confirmation by histopathological examination is mandatory. In contrast to the historical surgical approach, glucocorticosteroid therapy is currently considered first line therapy, in line with the RT currently being viewed as a manifestation of, or analogous to, IgG4-RSD. For disease relapse, immunomodulatory agents (azathioprine, methotrexate, rituximab) can be used.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Riedel thyroiditis (RT) (Morbus Riedel, Riedel Struma, Riedel goitre) was first described in 1886 by the German surgeon Bernhard Riedel, who reported on three patients treated by thyroidectomy at the International Congress of Surgery in 1894 and 1896 [1,2,3]; Riedel used the descriptive term ‘Eisenharte Struma’ (‘iron-hard goitre’) for the condition [1]. Iron-hard thyroiditis and struma lignose have then been used interchangeably. However, similar observations had been made by Semple already in 1864 and later by Bolby in 1888, who also used similar terminology (thyroid as hard as iron). Moreover, clinicians also appreciated the rare occurrence of a hard thyroid described as a ‘wooden’ or ‘stone’ goitre [1,2,3].
RT tends to affect individuals aged 30 to 60 years [4,5,6,7]. There is a gender predilection with females affected three times more often than males [1, 5,6,7,8]. Thyroidectomy has traditionally been performed for this condition [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. RT is a rare disease with an incidence of approximately 1:100,000 to 1.6:100,000 [38,39,40,41,42]. A comprehensive study conducted at the Mayo Clinic (from 1920 to 1984) identified 37 cases of RT among 57,000 thyroidectomies (0.06%) [7], but most of the literature corresponds to reports of individual cases (Table 1).
The aetiology of RT has been a topic of controversy, with genetic factors [50], viruses (e.g. Epstein-Barr) [51], and smoking [7] being raised and discussed as potential aetiological factors, but all lacking convincing evidence. More plausible is the notion that, RT likely represents an autoimmune process and a form of primary fibrogenic disease [4]. Similarities to Hashimoto’s thyroiditis and associations with other autoimmune diseases including Addison’s disease, type 1 diabetes mellitus, and pernicious anaemia have also been explored [52,53,54,55]. Currently, RT is regarded as a form of IgG4-related disease (IgG4-RSD) [56] and, in this context, may be referred to as IgG4-related sclerosing thyroiditis. Recently, Dahlgren et al. [57] attempted to advance the notion of a relationship between RT and IgG4-RSD; they examined tissues from three patients immunohistochemically and reported IgG4:IgG ratios ranging from 44–56% in two cases but only 0–20% in the remainder.
In RT, fibroblasts or fibroblast-like cells proliferate via the action of cytokines released from B- and/or T- lymphocytes [5]. Eosinophils may also have a role; degranulation of these cells has been described in RT [58], leading to ‘progressive fibrosis’ [7]. Eosinophil infiltration and extracellular MBP (major basic protein) deposition were observed by Heufelder et al. [58] in 15 of 16 patients with histologically proven Riedel’s invasive fibrous thyroiditis. Overall, the process has also been referred to as lymphoplasmacytosis with eosinophilia [59].
The fibroinflammatory process in RT involves not only the thyroid gland, but may also affect adjacent structures including parathyroids (hence frequently mimicking cT4 cancer clinically and on imaging) [59,60,61]; and may be accompanied by similar manifestations in organs known to be affected by the IgG4-RSD including orbital [50, 61,62,63], mediastinal/ thoracic (e.g., trachea, bronchi, lungs) [50, 64,65,66,67,68] and/or pancreatobiliary [7, 69] fibroinflammatory lesions. Bateman et al. [59] have also reported venous damage, which leads to phlebitis obliterans as seen in IgG4-RSD. Such systemic clinical settings are conveniently known as multifocal systemic sclerosis [50, 70].
The present article reviews the current knowledge about RT with emphasis on clinical presentation, diagnostics, and management.
Literature review
This review was based on a literature search conducted using the PubMed, Sinomed, Embase, Medline, Cochrane, Google Scholar and China National Knowledge Infrastructure databases and covering publications from 1896 to 2022. The following terms were used in connection with RT: ‘diagnose’, ‘glucocorticoid’, ‘IgG4-related systemic disease’ (IgG4-RSD), ‘Riedel struma’, ‘retroperitoneal fibrosis’, ‘tamoxifen’ and ‘treatment’.
Out of 137 articles identified during the search for various RT, ultimately 37 cases were included. Patients’ age ranged from 26 to 77 (median, 48). Individual results of this study are presented in Table 1.
Clinical presentation
RT manifests as a painless, hard, solid, ‘goitrous’ swelling in the mid-neck, causing tightness and trachea-oesophageal compression symptoms which may result in difficulty in breathing, dysphagia, hoarseness, aphonia, neck stiffness, coughing or a feeling of pressure [1, 5,6,7, 41, 42, 63, 71]. The symptoms are attributable to fibrosis which compresses and/or extends to the oesophagus, airways, recurrent laryngeal nerve, and musculature. Fibrosis of the parathyroid glands and ensuing hypoparathyroidism occur less frequently. About one-third of patients with RT suffer from ailments related to fibrosis in retroperitoneum/pancreas, mediastinum, lungs, lacrimal glands, orbit, salivary glands, and gallbladder [1, 7, 19, 39, 50,51,52,53,54,55,56,57, 59,60,61,62,63,64,65,66,67,68,69,70, 72, 73].
Occurrence of hypothyroidism varies. Percentages of about 30% have been reported [74], but the study conducted by the Mayo Clinic reported hypothyroidism in 14 (78%) of 19 patients. The remaining patients were euthyroid, but with elevated autoantibodies [antithyroglobulin (Tg-Abs) and antithyroid peroxidase (TPO-Abs) autoantibodies] [7]. Correlations between hypothyroidism and extent of fibrosis replacing the thyroid parenchyma are desirable.
Development of hyperthyroidism in the form of Graves’ disease [40, 63, 75, 76] or subacute thyroiditis [8, 30, 77, 78], in the course of RT are rare.
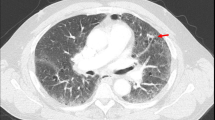
Clinically, it may be difficult to distinguish RT from Hashimoto’s thyroiditis or subacute thyroiditis due to comparable manifestation on imaging (Figs. 1a, b) [30, 35, 36, 40, 53, 63, 74, 76, 77, 79], and symptoms in RT may be similar to those in other thyroid diseases (Table 2) [40, 63, 75, 76].
Malignancy may co-exist with RT [40], including papillary thyroid carcinoma, anaplastic thyroid carcinoma [80], thyroid sarcoma [32], and lymphoma [79]. Hence, care should be taken not to overlook these diseases, as their clinical and gross the presentation may significantly overlap with that of RT.
Diagnosis
Laboratory tests
Initial blood tests should include assessment for thyroid diseases and autoimmune processes. Complete blood count, thyroid hormone evaluation (fT4, fT3, calcitonin), thyroid-stimulating hormone (TSH), TPO-Abs, Tg-Abs and TSHR are indicated.
An increased number of white blood cells, and, rarely, erythrocytopenia [38, 40] can be seen and results may be similar to those in Hashimoto’s thyroiditis [48, 74, 76, 81,82,83].
The relationship between RT and IgG4-RSD has been addressed above. IgG4-RSD can involve multiple organs, though rarely the thyroid gland [84]. It is characterized by a dense lymphoplasmacytic infiltrate (with increased IgG4( +) subpopulations), obliterative phlebitis and diffuse storiform fibrosis [85]. For the first time in 2001, Hamano et al. [73] observed that sclerosing pancreatitis was associated with high serum IgG4 levels and response to glucocorticoid therapy. Dahlgren et al. [57] suggested that IgG4-RSD, in addition to RT, is also associated with other diseases such as retroperitoneal fibrosis (pancreatitis) and Küttner tumour (also see summary below). Serum IgG4 concentrations are usually elevated to more than 135 mg/dL in IgG4-RSD, but this elevation is neither necessary (found in 75% or less of affected patients) nor sufficient for diagnosis of IgG4-RSD [57, 84].
Imaging
Ultrasonography and elastography
Ultrasonography reveals a diffuse, hypoechoic, ischaemic appearance, which is attributable to extensive fibrosis; hyperechoic bands correspond to the fibrosis [48, 74, 76, 81, 82].
Significant stiffness of the thyroid can be seen during ultrasound elastography [83].
99mTc thyroid scintigraphy
Isotope tests, such as thyroid scintigraphy using 99mTc, show no tracer uptake within the affected tissue.
Computed tomography and magnetic resonance imaging
Computed tomography (CT) shows hypodense areas within the thyroid gland, which remain unaltered after administration of a contrast agent (iodine dye) [48]. Additional imaging of the chest or abdomen may show involvement beyond the thyroid gland, indicative of a systemic process [48, 86].
Magnetic resonance imaging (MRI) reveals hypointense images by T1- and T2-weighted protocols [48].
A spectrum of slight to marked uniform enhancement can be observed following gadolinium administration [48, 82, 86,87,88].
Carotid artery encasement is characteristic and assists in differentiating from other thyroidopathies [7, 83].
Positron emission tomography (PET)
Positron emission tomography (PET) using [18F]fluoro-2-deoxy-D-glucose ([18F] FDG) clearly shows intense uptake where there are areas of inflammation–fibrosis in RT [83, 89, 90].
Fine needle aspiration (FNA), core and open biopsies
Thyroid FNA is often inconclusive and less helpful in RT compared to other thyroid diseases. The examination may show inflamed fibrous tissue, with a keloid-like appearance, but diagnostic features such as destruction of thyroid parenchyma, storiform fibrosis, and extrathyroidal extension are only seen on core needle or open biopsy samples and in FNA specimens. An elevated number of IgG4 ( +) plasma cells can be observed, but overall, the features are difficult to differentiate from other disorders with similar presentation like subacute thyroiditis, the fibrous subtype of Hashimoto thyroiditis or the paucicellular subtype of anaplastic thyroid carcinoma. An open biopsy is therefore often required and can be considered optimal.
Histopathology
Currently, histological examination remains the mainstay of the diagnosis and the decision to perform a biopsy is useful for the diagnosis of RT.
Resection specimens show replacement of most of or the whole thyroid by whitish poorly marginated hard fibrous tissue with variable elastic consistency and peripheral entrapment of brownish original thyroid tissue remnants and or adjacent periglandular soft tissue (Fig. 2). Microscopic examination shows thyroid tissue with architectural distortion due to the presence of extensive fibrosis, with severe atrophy of the follicles, dense inflammatory infiltrate, and abundant plasma cells (Figs. 3a, b, c, d). Overall, RT shows the key features of IgG4-RSD including tumefactive lymphoplasmacytic inflammation, prominent storiform fibrosis and frequent obliterative angiitis (mostly phlebitis). The coexistence of elevated serum IgG4 concentrations and their presence in the histopathological examination is necessary for the diagnosis of IgG4-RD. These features are included in the revised comprehensive diagnostic (RCD) criteria for IgG4-RD. The manifestation of Riedel's disease often meets all the IgG4-RD criteria, which may indicate the co-occurrence of these diseases [91]. Immunohistochemical evaluation for IgG and IgG4 assists in reaching a diagnosis of RT, with more than 80 IgG4 ( +) plasma cells/mm2 and an IgG4/IgG ratio greater than 40% [92]. However, similar to the basic features of IgG4-RSD in other organs, these histopathological features may vary greatly based on the age of the process, from a more fibrous, paucicellular fibroinflammatory reaction to the reverse. Accordingly, in advanced stages, the predominant histopathological findings are marked tissue storiform fibrosis, absence of thyroid follicles and poor, lymphocyte-predominant cellularity. The processes characteristically extend into the perithyroidal adipose tissue, vessels (even with associated thrombosis), nerves, and even the trachea and muscles.
a The thyroid gland parenchyma has been completely overtaken by asymmetrically distributed, variably concentrated, inflammatory cell infiltrates along with fibrosis (original magnification × 20). b A higher power shows bundled collagen interlacing at different angles (storiform fibrosis) and predominantly lymphoplasmacytic inflammatory infiltrates. An involved small nerve is discernible in the upper right quadrant (original magnification × 40). c There is marked (keloid-like) fibrosis in this area of the gland where no residual thyroid parenchyma is noted. There is a lymphoplasmacytic inflammatory aggregate in the lower left corner (high power) (original magnification × 40). d A muscular vessel showing lumen obliteration by fibrosis with inflammation (obliterative phlebitis) is in the upper left quadrant (original magnification × 40)
Management
Standards of care are not yet established for RT, but surgery and pharmacological treatments are considered.
Surgery
Several authors accept that surgical treatment is not indicated, at least initially [6, 7, 34]. However, particularly historically, total thyroidectomy has been attempted [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] to relieve compression symptoms [5, 34, 71]. Nonetheless, in the presence of significant extrathyroidal extension, surgery can be challenging and if the great vessels of the neck are encased (see above) may not be possible. After total thyroidectomy Levothyroxine is used as standard [22,23,24,25, 31, 33, 34].
If total thyroidectomy is not technically feasible, a decompressing isthmectomy maybe considered.
As the tissues in RT are very fibrous, surgical complications often occur (e.g., hypoparathyroidism or recurrence of compression symptoms) [13]. Calcium, along with calcitriol, can be usually included to counteract potential hypoparathyroidism [93].
Pharmacological treatment
The standard approach to suppress RT is the administration of both glucocorticoids [49, 52, 77, 94,95,96] and tamoxifen [97].
Glucocorticoids
Glucocorticoids are used to treat autoimmune thyroid disease and relieve symptoms of upper respiratory tract compression, dysphonia and inflammation of the laryngeal nerve [77, 94, 98]. Standard dosages are 100 mg of prednisone daily [5]. Administration can start with lower doses (from 15 to 60 mg) and even stay at these if the response is satisfactory [9,10,11,12,13, 15, 18, 19, 24, 27, 31, 33, 36, 43, 44, 46,47,48,49, 78, 79, 94, 96, 99]. In the case of smokers, the dose should be increased and the therapy repeated [7]. Glucocorticosteroid therapy is often effective, but it may be followed by relapses requiring the use of immunomodulatory agents such as azathioprine, methotrexate, and, recently, rituximab [100].
Tamoxifen
Tamoxifen is a non-steroidal selective oestrogen-receptor modulator (SERM) of the triphenylethylene family, which include clomifene, nafoxidine, ospemifene and toremifene [101, 102]; and is structurally derived from diethylstilbestrol-type oestrogens and antioestrogens, such as chlorotrianisene and ethamoxytriphetol. Clomiphene was synthesized initially and then tamoxifen was developed [103,104,105].
Side effects include an increase in triglyceride concentration, which may slightly increase the risk of pulmonary embolism, deep vein thrombosis, or stroke [68]. Cases of hepatotoxicity have been observed with a long-term use [106]. Tamoxifen may also cause non-alcoholic fatty liver disease in overweight and obese females [69].
Currently, no acute overdose of tamoxifen has been observed [10, 11, 15, 18, 19, 24, 31, 43, 45, 47, 78, 97, 107,108,109,110]. It is noted that tamoxifen is used in other inflammatory conditions related to multifocal fibrosis [108]. It should be mentioned that this drug is primarily administered in breast cancer [32, 41, 42], dysmenorrhoea [52], gynaecomastia [56, 111], infertility [55], and early puberty-like bone maturation (in cases of females with precocious puberty) [57, 112] and McCune-Albright syndrome [57, 64, 100].
Mycophenolate mofetil
Mycophenolate mofetil (MM) is the 2-morpholinoethyl ester of mycophenolic acid (MPA), which suppresses the immune system by cytostatically affecting T- and B-lymphocytes. MPA selectively and reversibly inhibits inosine monophosphate dehydrogenase, which is involved in the synthesis of the guanosine nucleosides necessary for the assembly of DNA. It does not, however, affect cytokine synthesis and does not reduce the activity of neutrophils [113]. MM is used to prevent acute rejection of organ transplants (heart, liver, kidney) in combination with cyclosporine and corticosteroids in allogeneic transplant recipients [114, 115]. MM is also used in RT in combination with rituximab, as MPA alone is too weak [13].
Other methods of treatment
Management of RT relapses after glucocorticosteroid therapy has been addressed above.
In the event of hyperthyroidism, radioiodine therapy or, in uncontrolled cases, external beam radiotherapy may be used [43], and levothyroxine is administered when hypothyroidism occurs [9, 10, 12, 16, 18, 20, 22,23,24,25, 27, 28, 31, 33, 34, 36, 47, 49, 99, 111].
There is little information on how vitamin D levels affect RT. However, because hypoparathyroidism is a side effect of RT, vitamin D is frequently mentioned in relation to the treatment. The adverse consequences of parathyroid hormone insufficiency are eliminated by using vitamin D and calcium [4, 77, 116,117,118].
Conclusion
RT is a rare disease affecting the thyroid gland and adjacent tissues, clinically frequently mimicking locally advanced (cT4) malignancy. The disease leads to gradual progressive fibrosis with compression symptoms, pain, and hypothyroidism. Extrathyroidal extension in the central neck can also lead to hypoparathyroidism and vocal cord palsy. Rarely, RT may be limited to the thyroid gland. Imaging with the use of ultrasonography [48, 74, 76, 81, 82], CT [7, 48, 86], and MRI [48] or PET [79, 81, 83] assists in assessing the extent of lesions in the thyroid and the presence of additional manifestations in other organs. Diagnosis may be difficult without biopsy and histopathological difficulties in differentiating RT from anaplastic carcinoma [80] or thyroid sarcomas are experienced [32].
Upon diagnosis of RT, it is important to search for other systemic fibrosing manifestations in IgG4-RSD target organs (parathyroid glands, salivary glands, lacrimal glands, trachea, nervous system, cardiovascular system, retroperitoneum, mediastinum, lungs, etc.). Immunohistochemistry is recommended to assess the extent of IgG4 ( +) plasma cell population. Clinical trials have shown that in nearly 95% of RT cases, there are increased serum concentrations of IgG4 antibodies [85]. Although serum IgG4 levels may have a valuable role in diagnosis and post-treatment monitoring, currently, serum IgG4 level is not regarded as a specific marker in diagnosis and management of RT. Further research is desirable to verify the sensitivity and specificity of this finding [93].
It should be emphasized that RT cannot be completely cured. Glucocorticoids (prednisone, prednisolone) continue to be the initial treatment of choice. This has an anti-inflammatory effect and reduces the size of the gland, allowing the relief of compressive symptoms. Glucocorticosteroid therapy is effective but may be followed by relapses requiring the use of immunomodulatory agents, such as azathioprine, methotrexate, and recently rituximab [11, 13, 113]. In patients with symptomatic fibro-inflammatory disease in a hypothyroid phase, levothyroxine therapy should be started, and in special cases, anti-inflammatory drugs and vitamin D should be administered [4, 77, 93, 116,117,118].
Data Availability
Data sharing not applicable.
References
Riedel BM (1896) Die chronische, zur Bildung eisenharter Tumoren fuhrende Entzundung der Schilddruse. Verh Dtsch Ges Chir 25:101–105
Riedel BM (1896) Vorstellung eines Kranken mit chronischer Strumitis. Verh Ges Chir 26:127–129
Riedel BM (1910) Ueber Verlauf und Ausgang der chronischer Strumitis. Munch Med Wochenschr 57:1946–1947
Majety P, Hennessey JV (2000) Acute and Subacute, and Riedel’s Thyroiditis. 2022 Jul 25. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Hofland J, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc
Guimaraes VC (2010) Subacute and Reidel's Thyroiditis. In: Jameson JL, De Groot LJ, eds. Endocrinology: Adult and Pediatric. Vol 2. 6th ed. Philadelphia: Elsevier 1600–1603
Singer PA (1991) Thyroiditis. Acute, subacute, and chronic. Med Clin North Am 75(1):61–77. https://doi.org/10.1016/s0025-7125(16)30472-2
Fatourechi MM, Hay ID, McIver B, Sebo TJ, Fatourechi V (2011) Invasive fibrous thyroiditis (Riedel thyroiditis): the Mayo Clinic experience, 1976–2008. Thyroid 21(7):765–772. https://doi.org/10.1089/thy.2010.0453
Kabalak T, Ozgen AG (2002) Familial occurrence of subacute thyroiditis. Endocr J 49(2):207–209. https://doi.org/10.1507/endocrj.49.207
Er-Rahali Y, Massine El Hammoumi M, Issouani J, Nfad CA, El Moussaoui S, Kabiri EH, Guerboub AA (2021) Reidel’s Thyroiditis, a Diagnostic and Management Challenge: A Case Report and Review of the Literature. Case Rep Endocrinol 2021:5185259. https://doi.org/10.1155/2021/5185259
Shafi AA, Saad NB, AlHarthi B (2020) Riedel’s thyroiditis as a diagnostic dilemma - A case report and review of the literature. Ann Med Surg (Lond) 25(52):5–9. https://doi.org/10.1016/j.amsu.2020.02.006
Mammen SV, Gordon MB (2019) Successful use of rituximab in a case of Riedel thyroiditis resistant to treatment with prednisone and tamoxifen. Aace Clin Case Rep 5(3):e218–e221. https://doi.org/10.4158/ACCR-2018-0352
Simões CA, Tavares MR, Andrade NMM, Uehara TM, Dedivitis RA, Cernea CR (2018) Does the Intensity of IGG4 Immunostaining Have a Correlation with the Clinical Presentation of Riedel’s Thyroiditis? Case Rep Endocrinol 16(2018):4101323. https://doi.org/10.1155/2018/4101323
Falhammar H, Juhlin CC, Barner C, Catrina SB, Karefylakis C, Calissendorff J (2018) Riedel’s thyroiditis: clinical presentation, treatment and outcomes. Endocrine 60(1):185–192. https://doi.org/10.1007/s12020-018-1526-3
Sakai Y, Imamura Y (2018) Case report: IgG4-related mass-forming thyroiditis accompanied by regional lymphadenopathy. Diagn Pathol 13(1):3. https://doi.org/10.1186/s13000-017-0681-9
Darouichi M, Constanthin PE (2016) Riedel’s thyroiditis. Radiol Case Rep 11(3):175–177. https://doi.org/10.1016/j.radcr.2016.05.017
Cai W, Kang H, Hai T (2016) Vasovagal reflex emergency caused by Riedel’s thyroiditis: A case report and review of the literature. Asian J Surg 39(1):41–44. https://doi.org/10.1016/j.asjsur.2013.01.008
Rajkovaca Z, Gajanin R, Pavkovic I, Kovacevic P, Kovacevic T (2016) A case of riedel’s thyroiditis. Acta Endocrinol (Buchar) 12(3):339–343. https://doi.org/10.4183/aeb.2016.339
Arowolo OA, Ige FS, Odujoko O, Agbakwuru EA (2016) Riedel’s thyroiditis in a black African: A case report and review of literature. Niger J Clin Pract 19(4):549–555. https://doi.org/10.4103/1119-3077.183311
Hakeem AH, Chandramathyamma SK, Hakeem IH, Wani FJ, Gomez R (2016) Riedel’s Thyroiditis Mimicking as Anaplastic Thyroid Carcinoma: Unusual Presentation. Indian J Surg Oncol 7(3):359–62. https://doi.org/10.1007/s13193-016-0513-5
Chong Xi R, Hong Qiao W, Yan L (2016) Severe trachea compression caused by Riedel’s thyroiditis: A case report and review of the literature. Ann Med Surg (Lond) 24(12):18–20. https://doi.org/10.1016/j.amsu.2016.10.005
Bhutia CT, Das D (2014) Riedel’s Thyroiditis in an Elderly Male Patient: A Rare Entity. J Clin Diagn Res 8(10):FD24-5. https://doi.org/10.7860/JCDR/2014/9215.5060
Hong JT, Lee JH, Kim SH, Hong SB, Nam M, Kim YS, Chu YC (2013) Case of concurrent Riedel’s thyroiditis, acute suppurative thyroiditis, and micropapillary carcinoma. Korean J Intern Med 28(2):236–41. https://doi.org/10.3904/kjim.2013.28.2.236
Lee DY, Moon JS, Kim GE, Kim HK, Kang HC (2013) Riedel thyroiditis in a patient with Graves disease. Endocrinol Metab (Seoul) 28(2):138–43. https://doi.org/10.3803/EnM.2013.28.2.138
Wang CJ, Wu TJ, Lee CT, Huang SM (2012) A misdiagnosed Riedel’s thyroiditis successfully treated by thyroidectomy and tamoxifen. J Formos Med Assoc 111(12):719–723. https://doi.org/10.1016/j.jfma.2012.07.012
Pi GY, Lee YS, Hong SW, Chang HS, Park CS (2012) A case of Riedel’s thyroiditis. J Korean Surg Soc 82(5):317–20. https://doi.org/10.4174/jkss.2012.82.5.317
Eryaman E, Comunoglu C (2011) Could Riedel’s thyroiditis be subacute thyroiditis? A case report. Pol J Pathol 62(3):176–178
Junik R, Juraniec O, Pypkowski J, Krymer A, Marszałek A (2011) A difficult diagnosis: a case report of combined Riedel’s disease and fibrosing Hashimoto’s thyroiditis. Endokrynol Pol 62(4):351–356
Pirola I, Morassi ML, Braga M, De Martino E, Gandossi E, Cappelli C (2009) A Case of Concurrent Riedel’s, Hashimoto’s and Acute Suppurative Thyroiditis. Case Rep Med 2009:535974. https://doi.org/10.1155/2009/535974
Won YS, Lee HH, Lee YS, Kim JS, Jeon HM, Jung SS, Lee JH, Park WC (2008) A case of Riedel’s thyroiditis associated with benign nodule: mimic of anaplastic transformation. Int J Surg 6(6):e24–e27. https://doi.org/10.1016/j.ijsu.2006.09.011
Cho MH, Kim CS, Park JS, Kang ES, Ahn CW, Cha BS, Lim SK, Kim KR, Lee HC (2007) Riedel’s thyroiditis in a patient with recurrent subacute thyroiditis: a case report and review of the literature. Endocr J 54(4):559–562. https://doi.org/10.1507/endocrj.k06-186
Dabelic N, Jukic T, Labar Z, Novosel SA, Matesa N, Kusic Z (2003) Riedel’s thyroiditis treated with tamoxifen. Croat Med J 44(2):239–241
Torres-Montaner A, Beltrán M, Romero de la Osa A, Oliva H (2001) Sarcoma of the thyroid region mimicking Riedel’s thyroiditis. J Clin Pathol 54(7):570–2. https://doi.org/10.1136/jcp.54.7.570
Laitt RD, Hubscher SG, Buckels JA, Darby S, Elias E (1992) Sclerosing cholangitis associated with multifocal fibrosis: a case report. Gut 33(10):1430–1432. https://doi.org/10.1136/gut.33.10.1430
Marín F, Araujo R, Páramo C, Lucas T, Salto L (1989) Riedel’s thyroiditis associated with hypothyroidism and hypoparathyroidism. Postgrad Med J 65(764):381–383. https://doi.org/10.1136/pgmj.65.764.381
Ward MJ, Davies D (1981) Riedel’s thyroiditis with invasion of the lungs. Thorax 36(12):956–957. https://doi.org/10.1136/thx.36.12.956
Kelly WF, Mashiter K, Taylor S, Joplin GF (1979) Riedel’s thyroiditis leading to severe but reversible pituitary failure. Postgrad Med J 55(641):194–198. https://doi.org/10.1136/pgmj.55.641.194
Turner-Warwick R, Nabarro JD, Doniach D (1966) Riedel’s thyroiditis and retroperitoneal fibrosis. Proc R Soc Med 59(7):596–8
Volpe R (1995) Subacute and sclerosing thyroiditis. In: De Groot LJ (ed) Endocrinology, 3rd edn. WB Saunders, Philadelphia, pp 742–751
LiVolsi VA, LoGerfo P (1981) Thyroiditis. CRC Press, Boca Raton, FL
Schwaegerle SM, Bauer TW, Esselstyn CB Jr (1988) Riedel’s thyroiditis. Am J Clin Pathol 90(6):715–722. https://doi.org/10.1093/ajcp/90.6.715
Hay ID (1985) Thyroiditis: a clinical update. Mayo Clin Proc 60(12):836–843. https://doi.org/10.1016/s0025-6196(12)64789-2
Beahrs OH, McConahey WM, Woolner LB (1957) Invasive fibrous thyroiditis (Riedel’s struma). J Clin Endocrinol Metab 17(2):201–220. https://doi.org/10.1210/jcem-17-2-201
Lawless A, Papachristos A, Robinson B, Sidhu S, Eade T (2022) Refractory Riedel’s thyroiditis managed with low dose radiotherapy. Rep Pract Oncol Radiother 27(3):591–592. https://doi.org/10.5603/RPOR.a2022.0033
Góralska M, Podlewska M, Żach M (2021) Riedel’s thyroiditis - difficulties in differentiating from thyroid cancer. Endokrynol Pol 72(4):418–419. https://doi.org/10.5603/EP.a2021.0057
Navarro-Sánchez V, Marín-Castañeda LA, Gallegos CA, Quiroz O, Ahumada-Ayala M (2020) IgG4-Related Fibrous Thyroiditis (Riedel’s Thyroiditis): A Case Report. Am J Case Rep 21:e928046. https://doi.org/10.12659/AJCR.928046
Pacella JC, Niwattisaiwong S, Newman D (2021) IgG4-Related Retroperitoneal Fibrosis: A Rare Association With Riedel’s Thyroiditis. Cureus 13(3):e13997. https://doi.org/10.7759/cureus.13997
Zakeri H, Kashi Z (2011) Variable Clinical Presentations of Riedel’s Thyroiditis: Report of Two Cases. Case Rep Med 2011:709264. https://doi.org/10.1155/2011/709264
Ozgen A, Cila A (2000) Riedel’s thyroiditis in multifocal fibrosclerosis: CT and MR imaging findings. AJNR Am J Neuroradiol 21(2):320–1
Vaidya B, Harris PE, Barrett P, Kendall-Taylor P (1997) Corticosteroid therapy in Riedel’s thyroiditis. Postgrad Med J 73(866):817–819. https://doi.org/10.1136/pgmj.73.866.817
Comings DE, Skubi KB, Van Eyes J, Motulsky AG (1967) Familial multifocal fibrosclerosis. Findings suggesting that retroperitoneal fibrosis, mediastinal fibrosis, sclerosing cholangitis, Riedel’s thyroiditis, and pseudotumor of the orbit may be different manifestations of a single disease. Ann Intern Med 66(5):884–92. https://doi.org/10.7326/0003-4819-66-5-884
Fontaine S, Gaches F, Lamant L, Uzan M, Bennet A, Caron P (2005) An unusual form of Riedel’s thyroiditis: a case report and review of the literature. Thyroid 15(1):85–88. https://doi.org/10.1089/thy.2005.15.85
Zimmermann-Belsing T, Feldt-Rasmussen U (1994) Riedel’s thyroiditis: an autoimmune or primary fibrotic disease? J Intern Med 235(3):271–274. https://doi.org/10.1111/j.1365-2796.1994.tb01071.x
Drury MI, Sweeney EC, Heffernan SJ (1974) Invasive fibrous (Riedel’s) thyroiditis. Ir Med J 67(14):388–390
Merrington WR (1948) Chronic thyroiditis; a case showing features of both Riedel’s and Hashimoto’s thyroiditis. Br J Surg 35(140):423–6. https://doi.org/10.1002/bjs.18003514015
Rose E, Royster HP (1961) Invasive fibrous thyroiditis (Riedel’s struma). JAMA 176:224–6. https://doi.org/10.1001/jama.1961.63040160008013
Li Y, Nishihara E, Kakudo K (2011) Hashimoto’s thyroiditis: old concepts and new insights. Curr Opin Rheumatol 23(1):102–107. https://doi.org/10.1097/BOR.0b013e328341378c
Dahlgren M, Khosroshahi A, Nielsen GP, Deshpande V, Stone JH (2010) Riedel’s thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis Care Res (Hoboken) 62(9):1312–1318. https://doi.org/10.1002/acr.20215
Heufelder AE, Goellner JR, Bahn RS, Gleich GJ, Hay ID (1996) Tissue eosinophilia and eosinophil degranulation in Riedel’s invasive fibrous thyroiditis. J Clin Endocrinol Metab 81(3):977–984. https://doi.org/10.1210/jcem.81.3.8772560
Bateman AC, Deheragoda MG (2009) IgG4-related systemic sclerosing disease - an emerging and under-diagnosed condition. Histopathology 55(4):373–383. https://doi.org/10.1111/j.1365-2559.2008.03217.x
Hostalet F, Hellin D, Ruiz J (2003) Tumefactive fibroinflammatory lesion of the head and neck treated with steroids: a case report. Eur Arch Otorhinolaryngol 260(4):229–231
Frankenthaler R, Batsakis JG, Suarez PA (1993) Tumefactive fibroinflammatory lesions of the head and neck. Ann Otol Rhinol Laryngol 102(6):481–482
Cheng AYY et al (2004) Tumefactive fibroinflammatory lesion of the nasal cavity followed by Riedel’s thyroiditis. J Otolaryngol 33(5):315–318
de Lange WE, Freling NJ, Molenaar WM, Doorenbos H (1989) Invasive fibrous thyroiditis (Riedel’s struma): a manifestation of multifocal fibrosclerosis? A case report with review of the literature. Q J Med 72(268):709–717
Rao CR, Ferguson GC, Kyle VN (1973) Retroperitoneal fibrosis associated with Riedel’s struma. Can Med Assoc J 108(8):1019–21
Barret NR (1958) Idiopathic mediastinal fibrosis. Br J Surg 46(197):207–218. https://doi.org/10.1002/bjs.18004619703
Husband P, Knudsen A (1976) Idiopathic cervical and retroperitoneal fibrosis: report of a case treated with steroids. Postgrad Med J 52(614):788–793. https://doi.org/10.1136/pgmj.52.614.788
Raphael HA, Beahrs OH, Woolner LB, Scholz DA (1966) Riedel’s struma associated with fibrous mediastinitis: report of a case. Mayo Clin Proc 41(6):375–382
Wold LE, Weiland LH (1983) Tumefactive fibro-inflammatory lesions of the head and neck. Am J Surg Pathol 7(5):477–482. https://doi.org/10.1097/00000478-198307000-00010
Dyk T (1957) Chronic recurrent pancreatitis with unusual anatomical picture (Riedel's tumor) in a 12-year-old boy. Pol Arch Med Wewn 27(8):1119–28. Polish
Bartholomew LG, Cain JC, Woolner LB, Utz DC, Ferris DO (1963) Sclerosing cholangitis: its possible association with Riedel’s struma and fibrous retroperitonitis. Report of two cases. N Engl J Med 269:8–12. https://doi.org/10.1056/NEJM196307042690102
Pearce EN, Farwell AP, Braverman LE (2003) Thyroiditis. N Engl J Med 348(26):2646–2655. https://doi.org/10.1056/NEJMra021194. Erratum.In:NEnglJMed.2003Aug7;349(6):620
Lewiński A, Choroba Riedla W, Interna Szczeklika (2019) Piotr Gajewski (red). Kraków: Medycyna Praktyczna, 2019, s. 1351–1352. ISBN 978–83–7430–591–4
Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K (2001) High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 344(10):732–738. https://doi.org/10.1056/NEJM200103083441005
Best TB, Munro RE, Burwell S, Volpé R (1991) Riedel’s thyroiditis associated with Hashimoto’s thyroiditis, hypoparathyroidism, and retroperitoneal fibrosis. J Endocrinol Invest 14(9):767–772. https://doi.org/10.1007/BF03347912
Heufelder AE, Hay ID (1994) Evidence for autoimmune mechanisms in the evolution of invasive fibrous thyroiditis (Riedel’s struma). Clin Investig 72(10):788–793. https://doi.org/10.1007/BF00180548
McIver B, Fatourechi MM, Hay ID, Fatourechi V (2010) Graves’ disease after unilateral Riedel’s thyroiditis. J Clin Endocrinol Metab 95(6):2525–2526. https://doi.org/10.1210/jc.2009-2609
Chopra D, Wool MS, Crosson A, Sawin CT (1978) Riedel’s struma associated with subacute thyroiditis, hypothyroidism, and hypoparathyroidism. J Clin Endocrinol Metab 46(6):869–871. https://doi.org/10.1210/jcem-46-6-869
Yasmeen T, Khan S, Patel SG, Reeves WA, Gonsch FA, de Bustros A, Kaplan EL (2002) Clinical case seminar: Riedel’s thyroiditis: report of a case complicated by spontaneous hypoparathyroidism, recurrent laryngeal nerve injury, and Horner’s syndrome. J Clin Endocrinol Metab 87(8):3543–3547. https://doi.org/10.1210/jcem.87.8.8752
Vigouroux C, Escourolle H, Mosnier-Pudar H, Thomopoulos P, Louvel A, Chapuis Y, Varet B, Luton JP (1996) Thyroïdite de Riedel et lymphome. Difficultés diagnostiques [Riedel's thyroiditis and lymphoma. Diagnostic difficulties]. Presse Med 25(1):28–30. French
Wan SK, Chan JK, Tang SK (1996) Paucicellular variant of anaplastic thyroid carcinoma. A mimic of Reidel’s thyroiditis. Am J Clin Pathol 105(4):388–93. https://doi.org/10.1093/ajcp/105.4.388
Lu L, Gu F, Dai WX, Li WY, Chen J, Xiao Y, Zeng ZP (2010) Clinical and pathological features of Riedel’s thyroiditis. Chin Med Sci J 25(3):129–134. https://doi.org/10.1016/s1001-9294(10)60036-3
Papi G, Corrado S, Cesinaro AM, Novelli L, Smerieri A, Carapezzi C (2002) Riedel’s thyroiditis: clinical, pathological and imaging features. Int J Clin Pract 56(1):65–7
Slman R, Monpeyssen H, Desarnaud S, Haroche J, Fediaevsky Ldu P, Fabrice M, Seret-Begue D, Amoura Z, Aurengo A, Leenhardt L (2011) Ultrasound, elastography, and fluorodeoxyglucose positron emission tomography/computed tomography imaging in Riedel’s thyroiditis: report of two cases. Thyroid 21(7):799–804. https://doi.org/10.1089/thy.2010.0242
Chetty R, Serra S, Gauchotte G, Märkl B, Agaimy A (2011) Sclerosing nodular lesions of the gastrointestinal tract containing large numbers of IgG4 plasma cells. Pathology 43(1):31–35. https://doi.org/10.1097/PAT.0b013e328340e450
Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R et al (2012) Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum 64:3061–3067
Pérez Fontán FJ, Cordido Carballido F, Pombo Felipe F, Mosquera Oses J, Villalba Martin C (1993) Riedel thyroiditis: US, CT, and MR evaluation. J Comput Assist Tomogr 17(2):324–5
Lo JC, Loh KC, Rubin AL, Cha I, Greenspan FS (1998) Riedel’s thyroiditis presenting with hypothyroidism and hypoparathyroidism: dramatic response to glucocorticoid and thyroxine therapy. Clin Endocrinol (Oxf) 48(6):815–818. https://doi.org/10.1046/j.1365-2265.1998.00449.x
Takahashi N, Okamoto K, Sakai K, Kawana M, Shimada-Hiratsuka M (2002) MR findings with dynamic evaluation in Riedel’s thyroiditis. Clin Imaging 26(2):89–91. https://doi.org/10.1016/s0899-7071(01)00373-4
Kotilainen P, Airas L, Kojo T, Kurki T, Kataja K, Minn H, Nuutila P (2004) Positron emission tomography as an aid in the diagnosis and follow-up of Riedel’s thyroiditis. Eur J Intern Med 15(3):186–189. https://doi.org/10.1016/j.ejim.2004.03.002
Drieskens O, Blockmans D, Van den Bruel A, Mortelmans L (2002) Riedel’s thyroiditis and retroperitoneal fibrosis in multifocal fibrosclerosis: positron emission tomographic findings. Clin Nucl Med 27(6):413–415. https://doi.org/10.1097/00003072-200206000-00005
Umehara H, Okazaki K, Kawa S, Takahashi H, Goto H, Matsui S, Ishizaka N, Akamizu T, Sato Y, Kawano M, Research Program for Intractable Disease by the Ministry of Health, Labor and Welfare (MHLW) Japan (2021) The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod Rheumatol 31(3):529–533. https://doi.org/10.1080/14397595.2020.1859710
Blanco VM, Páez CA, Victoria AM, Arango LG, Arrunategui AM, Escobar J, Martínez V, Guzmán GE (2019) Riedel’s Thyroiditis: Report of Two Cases and Literature Review. Case Rep Endocrinol 9(2019):5130106. https://doi.org/10.1155/2019/5130106
Hennessey JV (2011) Clinical review: Riedel’s thyroiditis: a clinical review. J Clin Endocrinol Metab 96(10):3031–3041. https://doi.org/10.1210/jc.2011-0617
Bagnasco M, Passalacqua G, Pronzato C, Albano M, Torre G, Scordamaglia A (1995) Fibrous invasive (Riedel’s) thyroiditis with critical response to steroid treatment. J Endocrinol Invest 18(4):305–307. https://doi.org/10.1007/BF03347818
Thomson JA, Jackson IM, Duguid WP (1968) The effect of steroid therapy on Riedel’s thyroiditis. Scott Med J 13(1):13–16. https://doi.org/10.1177/003693306801300103
Rodriguez I, Ayala E, Caballero C, De Miguel C, Matias-Guiu X, Cubilla AL, Rosai J (2001) Solitary fibrous tumor of the thyroid gland: report of seven cases. Am J Surg Pathol 25(11):1424–1428. https://doi.org/10.1097/00000478-200111000-00011
Few J, Thompson NW, Angelos P, Simeone D, Giordano T, Reeve T (1996) Riedel's thyroiditis: treatment with tamoxifen. Surgery 120(6):993–8; discussion 998–9. https://doi.org/10.1016/s0039-6060(96)80045-6
Brazier DJ, Sanders MD (1983) Multifocal fibrosclerosis associated with suprasellar and macular lesions. Br J Ophthalmol 67(5):292–296. https://doi.org/10.1136/bjo.67.5.292
Mansberg R, Bency R, Shen L, Bui C, Park K (2015) Riedel’s Thyroiditis with Intense FDG Uptake Demonstrated on FDG PET/CT. Mol Imaging Radionucl Ther 24(1):29–31. https://doi.org/10.4274/mirt.98598
Palazzo E, Palazzo C, Palazzo M (2014) IgG4-related disease. Joint Bone Spine 81(1):27–31. https://doi.org/10.1016/j.jbspin.2013.06.001
Khan MA, Hashmi SM, Prinsley PR, Premachandra DJ (2004) Reidel’s thyroiditis and Tolosa-Hunt syndrome, a rare association. J Laryngol Otol 118(2):159–161. https://doi.org/10.1258/002221504772784676
Meyer S, Hausman R (1976) Occlusive phlebitis in multifocal fibrosclerosis. Am J Clin Pathol 65(3):274–283. https://doi.org/10.1093/ajcp/65.3.274
Harach HR, Williams ED (1983) Fibrous thyroiditis–an immunopathological study. Histopathology 7(5):739–751. https://doi.org/10.1111/j.1365-2559.1983.tb02286.x
Hartemann P, Leclere J, Mizrahi R, Zannetti A, Genton P, Parache RM (1979) Un cas de thyroïdite de Riedel d'évolution regressive [A case of regressive Riedel's thyroiditis (author's transl)]. Ann Endocrinol (Paris) 40(1):65–6. French
Heufelder AE, Bahn RS (1994) Modulation of Graves’ orbital fibroblast proliferation by cytokines and glucocorticoid receptor agonists. Invest Ophthalmol Vis Sci 35(1):120–127
Hache L, Utz DC, Woolner LB (1962) Idiopathic fibrous retroperitonitis. Surg Gynecol Obstet 115:737–44
Jung YJ, Schaub CR, Rhodes R, Rich FA, Muehlenbein SJ (2004) A case of Riedel’s thyroiditis treated with tamoxifen: another successful outcome. Endocr Pract 10(6):483–6. https://doi.org/10.4158/EP.10.6.483
Clark CP, Vanderpool D, Preskitt JT (1991) The response of retroperitoneal fibrosis to tamoxifen. Surgery 109(4):502–506
Pritchyk K, Newkirk K, Garlich P, Deeb Z (2004) Tamoxifen therapy for Riedel’s thyroiditis. Laryngoscope 114(10):1758–1760. https://doi.org/10.1097/00005537-200410000-00015
De M, Jaap A, Dempster J (2001) Tamoxifen therapy in steroid resistant Reidel’s thyroiditis. Scott Med J 46(2):56–57. https://doi.org/10.1177/003693300104600211
Neild GH, Rodriguez-Justo M, Wall C, Connolly JO (2006) Hyper-IgG4 disease: report and characterisation of a new disease. BMC Med 6(4):23. https://doi.org/10.1186/1741-7015-4-23
Ross DS, Daniels GH (1992) Riedel’s thyroiditis associated with Hashimoto’s thyroiditis. J Endocrinol Invest 15(6):479. https://doi.org/10.1007/BF03348780
Allison AC, Eugui EM (2000) Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47(2–3):85–118. https://doi.org/10.1016/s0162-3109(00)00188-0
Budde K, Bunnapradist S, Grinyo JM, Ciechanowski K, Denny JE, Silva HT, Rostaing L, Envarsus study group (2014) Novel once-daily extended-release tacrolimus (LCPT) versus twice-daily tacrolimus in de novo kidney transplants: one-year results of Phase III, double-blind, randomized trial. Am J Transplant 14(12):2796–806. https://doi.org/10.1111/ajt.12955
Adam BA, Gebel HM (2020) IgE in Antibody-Mediated Rejection: A Novel Pathogenic Mechanism? Clin J Am Soc Nephrol 15(10):1392–1393. https://doi.org/10.2215/CJN.13000820
Boumans D, de Vries PA, Rikken NE, Laverman GD (2013) Prolonged hypocalcaemia after pamidronate infusion in Riedel’s thyroiditis associated hypoparathyroidism. Neth J Med 71(8):442–443
Czarnywojtek A, Florek E, Pietrończyk K, Sawicka-Gutaj N, Ruchała M, Ronen O, Nixon IJ, Shaha AR, Rodrigo JP, Tufano RP, Zafereo M, Randolph GW, Ferlito A (2023) The Role of Vitamin D in Autoimmune Thyroid Diseases: A Narrative Review. J Clin Med 12(4):1452. https://doi.org/10.3390/jcm12041452
Soh SB, Pham A, O’Hehir RE, Cherk M, Topliss DJ (2013) Novel use of rituximab in a case of Riedel’s thyroiditis refractory to glucocorticoids and tamoxifen. J Clin Endocrinol Metab 98(9):3543–3549. https://doi.org/10.1210/jc.2012-4050
Author information
Authors and Affiliations
Contributions
A.C., E.F. and K.P conception and design of the work; A.F., E.F., L.D.R.T., A.T and M.R. acquisition, analysis and interpretation of data; A.C., E.F. and K.P drafting the MS; A.F., L.D.R.T., A.T., N.S.-G., M.R., M.T.P., I.J.N., A.R.S., M.Z, G.W.R., P.A., A.A. and A.A.G. revising it critically for important intellectual content and scientific integrity. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Declarations
No tissue samples were analyzed for this study which is merely based on published literature.
Ethical approval
Ethical clearance is not applicable.
Conflicts of interest
The authors have no financial or non-financial conflicts of interest to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was written by members and invitees of the International Head and Neck Scientific Group (www.IHNSG.com).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Czarnywojtek, A., Pietrończyk, K., Thompson, L.D.R. et al. IgG4-related sclerosing thyroiditis (Riedel-Struma): a review of clinicopathological features and management. Virchows Arch 483, 133–144 (2023). https://doi.org/10.1007/s00428-023-03561-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-023-03561-2