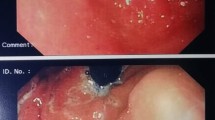
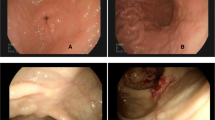
Abstract
Indolent T-cell lymphoproliferative disorder of the gastrointestinal tract (iTLPD-GI) is a rare neoplasm usually having an indolent clinical course and easily misdiagnosed as inflammatory bowel disease or other T-cell lymphomas. A subset of the disorders that progressed to overt peripheral T-cell lymphoma have been reported, and the etiology and pathogenesis are poorly understood. The current study retrospectively examined the pathological, molecular, and clinical features of 6 cases of iTLPD-GI. Hematoxylin and eosin staining, immunohistochemistry, in situ hybridization, T-cell receptor gene rearrangement, and next-generation sequencing (NGS) were performed with the diseased tissues. All the 6 patients were immunocompetent Chinese men, who presented with recurrent abdominal pain and diarrhea for 4 to 13 years. Histologically, the intestinal tissue was expanded by lymphoid infiltration, composed of small-to-medium-sized lymphocytes with gland intact. The neoplastic cells were CD4 − /CD8 + with expression of TIA1 and variable granzyme B in five cases, and the other one was CD4 + /CD8 − . Two of the 5 patients progressed to more aggressive T-cell lymphoma and died of disease with complications. NGS identified TET2 and DDX3X mutations in patient 1, and BIRC6 and REV3L mutations in patient 2. Literature review indicated that iTLPD-GI with CD4 − /CD8 + immunophenotype was more commonly reported in Chinese cases. Our limited data indicated CD4-/CD8 + iTLPD-GI have similar potential to progress to more aggressive T-cell lymphoma as that of CD4 + /CD8 − , and gradually increased expression of granzyme B and Ki-67 may be early signs of the disease progression. Gain of novel gene mutations may be indicators of the pathogenesis.





Similar content being viewed by others
Abbreviations
- CD:
-
Crohn disease
- CHOP:
-
Cyclophosphamide-hydroxydaunorubicin-oncovin-prednisone
- CISH:
-
Chromogenic in situ hybridization
- EATL–II:
-
Type II enteropathy-associated T-cell lymphoma
- EBER:
-
Epstein-Barr virus–encoded RNA
- FFPE:
-
Formalin-fixed, paraffin-embedded
- FISH:
-
Fluorescence in situ hybridization
- HPF:
-
High-power field; H&E, Hematoxylin and eosin
- IHC:
-
Immunohistochemistry
- iTLPD-GI:
-
Indolent T-cell lymphoproliferative disorder of the gastrointestinal tract
- IBD:
-
Inflammatory bowel disease
- ITCL NOS:
-
Intestinal T-cell lymphoma, not otherwise specified
- LI:
-
Labeling index
- MEITL:
-
Monomorphic epitheliotropic intestinal T-cell lymphoma
- NGS:
-
Next-generation sequencing
- PCR:
-
Polymerase chain reaction
- PTCL NOS:
-
Peripheral T-cell lymphoma, not otherwise specified
- TCR:
-
T-cell receptor
- UC:
-
Ulcerative colitis
- VAF:
-
Variant allele frequency
- WHO:
-
World Health Organization
References
Perry AM, Warnke RA, Hu Q et al (2013) Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood 122(22):3599–3606
Jaffe ES, Chott A, Ott G et al (2017) Intestinal T-cell lymphoma. In WHO classification of tumours haematopoietic and lymphoid tissues, Revised, 4th edition; WHO Classification of Tumours Editorial Board, Edition; IARC: Lyon, France
Carbonnel F, Lavergne A, Messing B et al (1994) Extensive small intestinal T-cell lymphoma of low-grade malignancy associated with a new chromosomal translocation. Cancer 73(4):1286–1291
Carbonnel F, d’Almagne H, Lavergne A et al (1999) The clinicopathological features of extensive small intestinal CD4 T cell infiltration. Gut 45(5):662–667
Margolskee E, Jobanputra V, Lewis SK, Alobeid B, Green PH, Bhagat G (2013) Indolent small intestinal CD4 + T-cell lymphoma is a distinct entity with unique biologic and clinical features. PLoS One 8(7):e68343
Sharma A, Oishi N, Boddicker RL et al (2018) Recurrent STAT-JAK2 fusions in indolent T-cell lymphoproliferative disorder of the gastrointestinal tract. Blood 131(20):2262–2266
Egawa N, Fukayama M, Kawaguchi K et al (1995) Relapsing oral and colonic ulcers with monoclonal T-cell infiltration. A low grade mucosal T-lymphoproliferative disease of the digestive tract. Cancer 75(7):1728–1733
Hirakawa K, Fuchigami T, Nakamura S et al (1996) Primary gastrointestinal T-cell lymphoma resembling multiple lymphomatous polyposis. Gastroenterology 111(3):778–782
Tsutsumi Y, Inada K, Morita K, Suzuki T (1996) T-cell lymphomas diffusely involving the intestine: report of two rare cases. Jpn J Clin Oncol 26(4):264–272
Isomoto H, Maeda T, Akashi T et al (2004) Multiple lymphomatous polyposis of the colon originating from T-cells: a case report. Dig Liver Dis 36(3):218–221
Zivny J, Banner BF, Agrawal S, Pihan G, Barnard GF (2004) CD4+ T-cell lymphoproliferative disorder of the gut clinically mimicking celiac sprue. Dig Dis Sci 49(4):551–555
Svrcek M, Garderet L, Sebbagh V et al (2007) Small intestinal CD4+ T-cell lymphoma: a rare distinctive clinicopathological entity associated with prolonged survival. Virchows Arch 451(6):1091–1093
Leventaki V, Manning JT Jr, Luthra R et al (2014) Indolent peripheral T-cell lymphoma involving the gastrointestinal tract. Hum Pathol 45(2):421–426
Sena Teixeira Mendes L, Attygalle AD, Cunningham D et al (2014) CD4-positive small T-cell lymphoma of the intestine presenting with severe bile-acid malabsorption: a supportive symptom control approach. Br J Haematol 167(2):265–269
Soon G, Wang S (2017) Indolent T-cell lymphoproliferative disease of the gastrointestinal tract in a renal transplant patient: diagnostic pitfalls and clinical challenges. Pathology 49(5):547–550
Edison N, Belhanes-Peled H, Eitan Y et al (2016) Indolent T-cell lymphoproliferative disease of the gastrointestinal tract after treatment with adalimumab in resistant Crohn’s colitis. Hum Pathol 57:45–50
Perry AM, Bailey NG, Bonnett M, Jaffe ES, Chan WC (2019) Disease progression in a patient with indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Int J Surg Pathol 27(1):102–107
Nagaishi T, Yamada D, Suzuki K et al (2019) Indolent T cell lymphoproliferative disorder with villous atrophy in small intestine diagnosed by single-balloon enteroscopy. Clin J Gastroenterol 12(5):434–440
Guo L, Wen Z, Su X, Xiao S, Wang Y (2019) Indolent T-cell lymphoproliferative disease with synchronous diffuse large B-cell lymphoma: a case report. Medicine (Baltimore) 98(17):e15323
Wang X, Ng CS, Chen C, Yu G, Yin W (2018) An unusual case report of indolent T-cell lymphoproliferative disorder with aberrant CD20 expression involving the gastrointestinal tract and bone marrow. Diagn Pathol 13(1):82
Soderquist CR, Patel N, Murty VV et al (2020) Genetic and phenotypic characterization of indolent T-cell lymphoproliferative disorders of the gastrointestinal tract. Haematologica 105(7):1895–1906
Shao SH, Gu HY, Lin DL, Shi HL, Zhang YJ, Li YJ (2019) Clinicopathological features of indolent T-cell lymphoproliferative disorder of the gastrointestinal tract: a report of five cases. Zhonghua Bing Li Xue Za Zhi 48(10):762–766
Kohri M, Tsukasaki K, Akuzawa Y et al (2020) Peripheral T-cell lymphoma with gastrointestinal involvement and indolent T-lymphoproliferative disorders of the gastrointestinal tract. Leuk Res 91:106336
Zanelli M, Zizzo M, Sanguedolce F et al (2020) Indolent T-cell lymphoproliferative disorder of the gastrointestinal tract: a tricky diagnosis of a gastric case. BMC Gastroenterol 20(1):336
Wu J, Li LG, Zhang XY et al (2020) Indolent T cell lymphoproliferative disorder of the gastrointestinal tract: an uncommon case with lymph node involvement and the classic Hodgkin’s lymphoma. J Gastrointest Oncol 11(4):812–819
Takahashi N, Tsukasaki K, Kohri M et al (2020) Indolent T-cell lymphoproliferative disorder of the stomach successfully treated by radiotherapy. J Clin Exp Hematop 60(1):7–10
Saggini A, Baciorri F, Di Prete M, Zizzari AG, Anemona L (2020) Oral manifestation of indolent T-cell lymphoproliferative disorder of the gastrointestinal tract: a potential diagnostic pitfall. J Cutan Pathol 47(5):494–496
Weng CY, Ye C, Fan YH, Lv B, Zhang CL, Li M (2022) CD8-positive indolent T-Cell lymphoproliferative disorder of the gastrointestinal tract: a case report and review of literature. World J Clin Cases 10(15):4971–4984
Swerdlow SH, Jaffe ES, Brousset P, Chan JK, de Leval L, Gaulard P, Harris NL, Pileri S, Weiss LM, International Lymphoma Study Group (2014) Cytotoxic T-cell and NK-cell lymphomas: current questions and controversies. Am J Surg Pathol 38(10):e60-71
Swerdlow SH, Campo E, Harris NL et al (2008) WHO classification of tumors of the haematopoietic and lymphoid tissues; IARC: Lyon, France
Yao WQ, Wu F, Zhang W et al (2020) Angioimmunoblastic T-cell lymphoma contains multiple clonal T-cell populations derived from a common TET2 mutant progenitor cell. J Pathol 250(3):346–357
Gong C, Krupka JA, Gao J et al (2021) Sequential inverse dysregulation of the RNA helicases DDX3X and DDX3Y facilitates MYC-driven lymphomagenesis. Mol Cell 81(19):4059–4075
Jiang Lu, Zhao-Hui Gu, Zi-Xun Y et al (2015) Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat Genet 47(9):1061–1066
Maura F, Agnelli L, Leongamornlert D et al (2019) Integration of transcriptional and mutational data simplifies the stratification of peripheral T-cell lymphoma. Am J Hematol 94(6):628–634
Montes-Mojarro IA, Chen BJ, Ramirez-Ibarguen AF et al (2020) Mutational profile and EBV strains of extranodal NK/T-cell lymphoma, nasaltype in Latin America. Mod Pathol 33(5):781–791
Ikeda F (2018) The anti-apoptotic ubiquitin conjugating enzyme BIRC6/BRUCE regulates autophagosome-lysosome fusion. Autophagy 14(7):1283–1284
Jiang TX, Zou JB, Zhu QQ et al (2019) SIP/CacyBP promotes autophagy by regulating levels of BRUCE/Apollon, which stimulates LC3-I degradation. Proc Natl Acad Sci U S A 116(27):13404–13413
Sarkozy C, Hung SS, Chavez EA et al (2021) Mutational landscape of gray zone lymphoma. Blood 137(13):1765–1776
Mosquera Orgueira A, Cid López M, Peleteiro Raíndo A et al (2021) Detection of Rare germline variants in the genomes of patients with B-cell neoplasms. Cancers (Basel) 13(6):1340
Wittschieben JP, Patil V, Glushets V, Robinson LJ, Kusewitt DF, Wood RD (2010) Loss of DNA polymerase zeta enhances spontaneous tumorigenesis. Cancer Res 70(7):2770–2778
Magro F, Langner C, Driessen A et al (2013) European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 7(10):827–851
Schofield JB, Haboubi N (2020) Histopathological mimics of inflammatory bowel disease. Inflamm Bowel Dis 26(7):994–1009
van Vliet C, Spagnolo DV (2020) T- and NK-cell lymphoproliferative disorders of the gastrointestinal tract: review and update. Pathology 52(1):128–141
Xie J, Huang Y, Zheng Y et al (2019) Acute Epstein-Barr virus positive cytotoxic T cell lymphoid hyperplasia in the upper aerodigestive tract, mimicking extranodal natural killer/T cell lymphoma, nasal type. Virchows Arch 474(2):219–226
Liu R, Wang M, Zhang L et al (2019) The clinicopathologic features of chronic active Epstein-Barr virus infective enteritis. Mod Pathol 32(3):387–395
Alaggio R, Amador C, Anagnostopoulos I et al (2022) The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia 3(7):1720–1748
Acknowledgements
Part of this study had been presented as a poster at the 11th Asia Pacific International Academy of Pathology Congress on October 9 to 14, 2019 in Hefei, China. We thank Dr. Qinglong Hu (Pathology Associates, Clovis, CA, USA) for his critical discussion and revision on the manuscript. We thank Dr. Shu-Yuan Xiao (Department of Pathology, The University of Chicago, Chicago, IL, USA) for his polishing the manuscript. We thank all investigators at Zhongnan Hospital of Wuhan University for participating in this study.
Funding
This study was supported by Zhongnan Hospital of Wuhan University, Science, Technology and Innovation Seed Fund (Project number: znpy2019067).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design.
Wei Fan carried out the testing of FISH, TCR, NGS, and analyzed the data. Li Niu carried out immunohistochemical staining. Huihua He and Jingping Yuan contributed case 2 (surgical resected specimen). Xueying Shi and Ye Wang contributed case 4, and Fei Yuan contributed case 5. Qiongrong Chen, Jingping Yuan, Xueying Shi, and Fei Yuan reviewed and analyzed all the 6 cases. Min Chen, Meifang Huang, Fuling Zhou, and Ye Wang collected and organized the clinical and endoscopic data. Jian Xu carried out the H&E staining and EBER. Qiongrong Chen coordinated the study and wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study has been conducted according to the guidelines of the local ethical committee (the clinical research ethics certificate number was 2021052 K).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material

Fig. S1
Single nucleotide variants tested by NGS on resected sample of patient 1. a TET2 mutation: T was inserted at codon 2482 in exon 3 of the TET2 gene (TET2 c.2482dupT); b Another point mutation of TET2: T was deleted at codon 2647 in exon 3 of the TET2 gene (TET2 c.2649delT). c Missense mutation [c.664G > C (p.A222P)] was detected in exon 7 of DDX3X gene (DDX3X c.664G > C) (PNG 385 KB).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, W., Niu, L., He, H. et al. Indolent T-cell lymphoproliferative disorder of gastrointestinal tract with unusual clinical courses: report of 6 cases and literature review. Virchows Arch 482, 729–743 (2023). https://doi.org/10.1007/s00428-022-03467-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-022-03467-5