Abstract
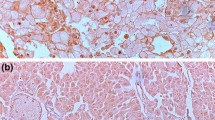
We previously reported that claspin is a key regulator in the progression of gastric cancer and renal cell carcinoma. However, the clinicopathological significance of claspin in urothelial carcinoma (UC) has not been investigated. We analyzed the expression and distribution of claspin in UC cases by immunohistochemistry. In the non-neoplastic urothelium, the expression of claspin was either weak or absent, whereas UC tissues showed nuclear staining. The expression of claspin was detected in 58 (42%) of a total of 138 upper tract UC cases treated by radical nephroureterectomy without neoadjuvant chemotherapy. Claspin-positive UC cases were associated with nodular/flat morphology, variant histology, high tumor grade, high pathological T grade, and lymphatic and venous invasion. The expression of claspin was significantly associated with decreased progression-free survival and cancer-specific survival. In addition, claspin was co-expressed with Ki-67, PD-L1, HER2, EGFR, and p53 in consecutive tumor sections of UC. An immunohistochemical analysis of claspin in biopsy specimens revealed that strong to moderate claspin staining was more frequently observed in carcinoma in situ in comparison to dysplasia or the benign urothelium. Furthermore, immunocytochemistry for claspin on urine cytology slides demonstrated that the proportion of claspin-positive cells was significantly greater in high-grade UC than in benign cases. These results suggest that claspin may be a novel prognostic marker and a possible therapeutic target molecule for UC. Moreover, claspin could be a useful diagnostic biomarker of urothelial neoplasia.




Similar content being viewed by others
Availability of data and material
All research data and material will be made available upon request. Most data are included in the main manuscript.
References
Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, Burger M, Cowan NC, Gontero P, Van Rhijn BWG, Mostafid AH, Palou J, Shariat SF (2018) European Association of Urology Guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol 73:111–122. https://doi.org/10.1016/j.eururo.2017.07.036
Leow JJ, Chong KT, Chang SL, Bellmunt J (2016) Upper tract urothelial carcinoma: a different disease entity in terms of management. ESMO Open 1:e000126. https://doi.org/10.1136/esmoopen-2016-000126
Abouassaly R, Alibhai SM, Shah N, Timilshina N, Fleshner N, Finelli A (2010) Troubling outcomes from population-level analysis of surgery for upper tract urothelial carcinoma. Urology 76:895–901. https://doi.org/10.1016/j.urology.2010.04.020
Jeldres C, Sun M, Isbarn H, Lughezzani G, Budäus L, Alasker A, Shariat SF, Lattouf JB, Widmer H, Pharand D, Arjane P, Graefen M, Montorsi F, Perrotte P, Karakiewicz PI (2010) A population-based assessment of perioperative mortality after nephroureterectomy for upper-tract urothelial carcinoma. Urology 75:315–320. https://doi.org/10.1016/j.urology.2009.10.004
Rebouissou S, Bernard-Pierrot I, de Reyniès A, Lepage ML, Krucker C, Chapeaublanc E, Hérault A, Kamoun A, Caillault A, Letouzé E, Elarouci N, Neuzillet Y, Denoux Y, Molinié V, Vordos D, Laplanche A, Maillé P, Soyeux P, Ofualuka K, Reyal F, Biton A, Sibony M, Paoletti X, Southgate J, Benhamou S, Lebret T, Allory Y, Radvanyi F (2014) EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci Transl Med 6:244ra291. https://doi.org/10.1126/scitranslmed.3008970
Zangouei AS, Barjasteh AH, Rahimi HR, Mojarrad M, Moghbeli M (2020) Role of tyrosine kinases in bladder cancer progression: an overview. Cell Commun Signal 18:127. https://doi.org/10.1186/s12964-020-00625-7
Siefker-Radtke AO, Apolo AB, Bivalacqua TJ, Spiess PE, Black PC (2018) Immunotherapy with checkpoint blockade in the treatment of urothelial carcinoma. J Urol 199:1129–1142. https://doi.org/10.1016/j.juro.2017.10.041
Tripathi A, Plimack ER (2018) Immunotherapy for urothelial carcinoma: current evidence and future directions. Curr Urol Rep 19:109. https://doi.org/10.1007/s11934-018-0851-7
Tomiyama E, Fujita K, Rodriguez Pena MDC, Taheri D, Banno E, Kato T, Hatano K, Kawashima A, Ujike T, Uemura M, Takao T, Yamaguchi S, Fushimi H, Yoshimura K, Uemura H, Netto GJ, Nonomura N (2020) Expression of nectin-4 and PD-L1 in upper tract urothelial carcinoma. Int J Mol Sci 21. https://doi.org/10.3390/ijms21155390
Favaretto RL, Zequi SC, Oliveira RAR, Santana T, Costa WH, Cunha IW, Guimarães GC (2018) Tissue-based molecular markers in upper tract urothelial carcinoma and their prognostic implications. Int Braz J Urol 44:22–37. https://doi.org/10.1590/S1677-5538.IBJU.2017.0204
Stone L (2017) Immunology: PD-1 and PD-L1 prognostic in UTUC. Nat Rev Urol 14:518. https://doi.org/10.1038/nrurol.2017.120
Fang D, Xiong GY, Li XS, Chen XP, Zhang L, Yao L, He ZS, Zhou LQ (2014) Pattern and risk factors of intravesical recurrence after nephroureterectomy for upper tract urothelial carcinoma: a large Chinese center experience. J Formos Med Assoc 113:820–827. https://doi.org/10.1016/j.jfma.2013.11.004
McKenney JK, Desai S, Cohen C, Amin MB (2001) Discriminatory immunohistochemical staining of urothelial carcinoma in situ and non-neoplastic urothelium: an analysis of cytokeratin 20, p53, and CD44 antigens. Am J Surg Pathol 25:1074–1078. https://doi.org/10.1097/00000478-200108000-00013
Asgari M, Nabi Maybodi M, Abolhasani M (2016) Differential diagnosis of urothelial carcinoma in situ from non-neoplastic urothelia: analysis of CK20, CD44, P53 and Ki67. Med J Islam Repub Iran 30:400
Tschirdewahn S, Vom Dorp F (2015) Diagnostics of nonmuscle-invasive urothelial cell carcinoma of the bladder. Urologe A 54:480–483. https://doi.org/10.1007/s00120-015-3772-9
Zhou AG, Hutchinson LM, Cosar EF (2014) Urine cytopathology and ancillary methods. Surg Pathol Clin 7:77–88. https://doi.org/10.1016/j.path.2013.10.003
Gopalakrishna A, Longo TA, Fantony JJ, Owusu R, Foo WC, Dash R, Inman BA (2016) The diagnostic accuracy of urine-based tests for bladder cancer varies greatly by patient. BMC Urol 16:30. https://doi.org/10.1186/s12894-016-0147-5
Abdullah LS (2013) The value of urine cytology in the diagnosis of bladder cancer. Cytopathological correlation Saudi Med J 34:937–941
Yafi FA, Brimo F, Steinberg J, Aprikian AG, Tanguay S, Kassouf W (2015) Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol Oncol 33:66.e25–31. https://doi.org/10.1016/j.urolonc.2014.06.008
Chang S, Smith E, Levin M, Rao JY, Moatamed NA (2015) Comparative study of ProEx C immunocytochemistry and UroVysion fluorescent in-situ hybridization assays on urine cytology specimens. Cytojournal 12:2. https://doi.org/10.4103/1742-6413.149845
Sullivan PS, Nooraie F, Sanchez H, Hirschowitz S, Levin M, Rao PN, Rao J (2009) Comparison of ImmunoCyt, UroVysion, and urine cytology in detection of recurrent urothelial carcinoma: a “split-sample” study. Cancer 117:167–173. https://doi.org/10.1002/cncy.20026
Comploj E, Mian C, Ambrosini-Spaltro A, Dechet C, Palermo S, Trenti E, Lodde M, Horninger W, Pycha A (2013) uCyt+/ImmunoCyt and cytology in the detection of urothelial carcinoma: an update on 7422 analyses. Cancer Cytopathol 121:392–397. https://doi.org/10.1002/cncy.21287
Dimashkieh H, Wolff DJ, Smith TM, Houser PM, Nietert PJ, Yang J (2013) Evaluation of UroVysion and cytology for bladder cancer detection: a study of 1835 paired urine samples with clinical and histologic correlation. Cancer Cytopathol 121:591–597. https://doi.org/10.1002/cncy.21327
Chini CC, Chen J (2004) Claspin, a regulator of Chk1 in DNA replication stress pathway. DNA Repair (Amst) 3:1033–1037. https://doi.org/10.1016/j.dnarep.2004.03.001
Chini CC, Chen J (2003) Human claspin is required for replication checkpoint control. J Biol Chem 278:30057–30062. https://doi.org/10.1074/jbc.M301136200
Jeong SY, Kumagai A, Lee J, Dunphy WG (2003) Phosphorylated claspin interacts with a phosphate-binding site in the kinase domain of Chk1 during ATR-mediated activation. J Biol Chem 278:46782–46788. https://doi.org/10.1074/jbc.M304551200
Benevolo M, Musio A, Vocaturo A, Donà MG, Rollo F, Terrenato I, Carosi M, Pescarmona E, Vocaturo G, Mottolese M (2012) Claspin as a biomarker of human papillomavirus-related high grade lesions of uterine cervix. J Transl Med 10:132. https://doi.org/10.1186/1479-5876-10-132
Bianco JN, Bergoglio V, Lin YL, Pillaire MJ, Schmitz AL, Gilhodes J, Lusque A, Mazières J, Lacroix-Triki M, Roumeliotis TI, Choudhary J, Moreaux J, Hoffmann JS, Tourrière H, Pasero P (2019) Overexpression of claspin and timeless protects cancer cells from replication stress in a checkpoint-independent manner. Nat Commun 10:910. https://doi.org/10.1038/s41467-019-08886-8
Ito F, Yoshimoto C, Yamada Y, Sudo T, Kobayashi H (2018) The HNF-1β-USP28-Claspin pathway upregulates DNA damage-induced Chk1 activation in ovarian clear cell carcinoma. Oncotarget 9:17512–17522. https://doi.org/10.18632/oncotarget.24776
Lin SY, Li K, Stewart GS, Elledge SJ (2004) Human claspin works with BRCA1 to both positively and negatively regulate cell proliferation. Proc Natl Acad Sci U S A 101:6484–6489. https://doi.org/10.1073/pnas.0401847101
Tsimaratou K, Kletsas D, Kastrinakis NG, Tsantoulis PK, Evangelou K, Sideridou M, Liontos M, Poulias I, Venere M, Salmas M, Kittas C, Halazonetis TD, Gorgoulis VG (2007) Evaluation of claspin as a proliferation marker in human cancer and normal tissues. J Pathol 211:331–339. https://doi.org/10.1002/path.2095
Kobayashi G, Sentani K, Hattori T, Yamamoto Y, Imai T, Sakamoto N, Kuraoka K, Oue N, Sasaki N, Taniyama K, Yasui W (2019) Clinicopathological significance of claspin overexpression and its association with spheroid formation in gastric cancer. Hum Pathol 84:8–17. https://doi.org/10.1016/j.humpath.2018.09.001
Kobayashi G, Sentani K, Babasaki T, Sekino Y, Shigematsu Y, Hayashi T, Oue N, Teishima J, Matsubara A, Sasaki N, Yasui W (2020) Claspin overexpression is associated with high-grade histology and poor prognosis in renal cell carcinoma. Cancer Sci 111:1020–1027. https://doi.org/10.1111/cas.14299
Li J, Lv B, Li X, He Z, Zhou K (2013) Apoptosis-related molecular differences for response to tyrosine kinase inhibitors in drug-sensitive and drug-resistant human bladder cancer cells. J Cancer Res Ther 9:668–671. https://doi.org/10.4103/0973-1482.126478
Zajac M, Ye J, Mukhopadhyay P, Jin X, Ben Y, Antal J, Gupta AK, Rebelatto MC, Williams JA, Walker J (2020) Optimal PD-L1-high cutoff for association with overall survival in patients with urothelial cancer treated with durvalumab monotherapy. PLoS ONE 15:e0231936. https://doi.org/10.1371/journal.pone.0231936
Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, Bamias A, Lebret T, Shariat SF, Park SH, Ye D, Agerbaek M, Enting D, McDermott R, Gajate P, Peer A, Milowsky MI, Nosov A, Neif Antonio J, Tupikowski K, Toms L, Fischer BS, Qureshi A, Collette S, Unsal-Kacmaz K, Broughton E, Zardavas D, Koon HB, Galsky MD (2021) Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med 384:2102–2114. https://doi.org/10.1056/NEJMoa2034442
Hattori T, Sentani K, Naohide O, Sakamoto N, Yasui W (2017) Clinicopathological significance of SPC18 in colorectal cancer: SPC18 participates in tumor progression. Cancer Sci 108:143–150. https://doi.org/10.1111/cas.13121
Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA, Akbani R, Castro MAA, Gibb EA, Kanchi RS, Gordenin DA, Shukla SA, Sanchez-Vega F, Hansel DE, Czerniak BA, Reuter VE, Su X, de Sa CB, Chagas VS, Mungall KL, Sadeghi S, Pedamallu CS, Lu Y, Klimczak LJ, Zhang J, Choo C, Ojesina AI, Bullman S, Leraas KM, Lichtenberg TM, Wu CJ, Schultz N, Getz G, Meyerson M, Mills GB, McConkey DJ, Weinstein JN, Kwiatkowski DJ, Lerner SP, Network TR (2017) Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 171:540-556.e525. https://doi.org/10.1016/j.cell.2017.09.007
Challita-Eid PM, Satpayev D, Yang P, An Z, Morrison K, Shostak Y, Raitano A, Nadell R, Liu W, Lortie DR, Capo L, Verlinsky A, Leavitt M, Malik F, Aviña H, Guevara CI, Dinh N, Karki S, Anand BS, Pereira DS, Joseph IB, Doñate F, Stover DR (2016) Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res 76:3003–3013. https://doi.org/10.1158/0008-5472.CAN-15-1313
Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK (2012) Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets 16:15–31. https://doi.org/10.1517/14728222.2011.648617
Siddiqui MR, Railkar R, Sanford T, Crooks DR, Eckhaus MA, Haines D, Choyke PL, Kobayashi H, Agarwal PK (2019) Targeting epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) expressing bladder cancer using combination photoimmunotherapy (PIT). Sci Rep 9:2084. https://doi.org/10.1038/s41598-019-38575-x
Okita R, Maeda A, Shimizu K, Nojima Y, Saisho S, Nakata M (2017) PD-L1 overexpression is partially regulated by EGFR/HER2 signaling and associated with poor prognosis in patients with non-small-cell lung cancer. Cancer Immunol Immunother 66:865–876. https://doi.org/10.1007/s00262-017-1986-y
Padmanabhan R, Kheraldine HS, Meskin N, Vranic S, Al Moustafa AE (2020) Crosstalk between HER2 and PD-1/PD-L1 in breast cancer: from clinical applications to mathematical models. Cancers (Basel) 12. https://doi.org/10.3390/cancers12030636
Chen M, Sharma A, Lin Y, Wu Y, He Q, Gu Y, Xu ZP, Monteiro M, Gu W (2019) Insulin and epithelial growth factor (EGF) promote programmed death ligand 1(PD-L1) production and transport in colon cancer stem cells. BMC Cancer 19:153. https://doi.org/10.1186/s12885-019-5364-3
Mallofré C, Castillo M, Morente V, Solé M (2003) Immunohistochemical expression of CK20, p53, and Ki-67 as objective markers of urothelial dysplasia. Mod Pathol 16:187–191. https://doi.org/10.1097/01.MP.0000056628.38714.5D
Courtade-Saïdi M, Aziza J, d’Aure D, Bérard E, Evrard S, Basset C, Lacoste-Collin L (2016) Immunocytochemical staining for p53 and Ki-67 helps to characterise urothelial cells in urine cytology. Cytopathology 27:456–464. https://doi.org/10.1111/cyt.12332
Acknowledgements
We thank Shinichi Norimura and Yuka Yamaguchi for the excellent technical assistance, and the Analysis Center of Life Science, Hiroshima University, for the use of its facilities.
Funding
This work was supported by Grants-in-Aid for Scientific Research (19K07434) from the Japan Society for the Promotion of Science. The Takeda Science Foundation also supported this work.
Author information
Authors and Affiliations
Contributions
Go Kobayashi: conceptualization, methodology, formal analysis, investigation, data curation, project administration, writing–original draft and writing-review and editing. Tetsutaro Hayashi: conceptualization, data curation, project administration, resources, and writing—review and editing. Kazuhiro Sentani: conceptualization, project administration, supervision, and writing—review and editing. Takashi Babasaki: conceptualization and writing—review and editing. Yohei Sekino: conceptualization and writing-review and editing. Shogo Inoue: conceptualization and writing—review and editing. Naohiro Uraoka: conceptualization and writing—review and editing. Masanori Hanamoto: conceptualization and writing-review and editing. Hiroyuki Nose: conceptualization and writing—review and editing. Jun Teishima: conceptualization and writing-review and editing. Naohide Oue: conceptualization and writing—review and editing. Akio Matsubara: conceptualization and writing—review and editing. Naomi Sasaki: conceptualization and writing—review and editing. Wataru Yasui: conceptualization and writing—review and editing. All authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
A statement on ethics approval and consent is in sect. “Materials and methods.”
Consent for publication
All authors have approved the final version of the manuscript and have given their consent for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kobayashi, G., Hayashi, T., Sentani, K. et al. Clinicopathological significance of claspin overexpression and its efficacy as a novel biomarker for the diagnosis of urothelial carcinoma. Virchows Arch 480, 621–633 (2022). https://doi.org/10.1007/s00428-021-03239-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03239-7