Abstract
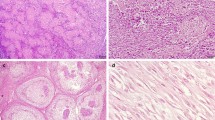
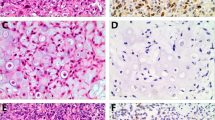
The aim of this study is to assess the usefulness of beta-catenin immunohistochemical expression in the differential diagnosis of osteoid-producing primary tumors of bone. Seventy cases of osteoid-producing tumors of bone (24 conventional osteosarcomas, 18 osteoblastomas, 13 osteoblastoma-like osteosarcomas, 10 chondroblastomas, and 5 chondroblastoma-like osteosarcomas) diagnosed at Istituto Ortopedico Rizzoli were reviewed and evaluated for the intensity, extension, and subcellular distribution of immunohistochemical expression of beta-catenin. A majority of cases (73%, 51 cases) exhibited cytoplasmic and/or membranous positivity in varied degrees of intensity and proportion of positive cells, in the absence of nuclear staining. Fifteen cases (21%) were completely negative, including two osteoblastomas, five chondroblastomas, three conventional osteosarcomas, four osteoblastoma-like osteosarcomas, and one chondroblastoma-like osteosarcoma. A minority of cases (6%) including three osteoblastoma-like osteosarcomas and one osteoblastoma showed focal nuclear beta-catenin positivity with or without concomitant cytoplasmic staining. In the current series, beta-catenin showed not to be useful in the differential diagnosis of osteoid-producing primary bone tumors.


Similar content being viewed by others
References
Gambarotti M, Dei Tos AP, Vanel D, Picci P, Gibertoni D, Klein MJ, Righi A (2019) Osteoblastoma-like osteosarcoma: high-grade or low-grade osteosarcoma? Histopathology 74:494–503. https://doi.org/10.1111/his.13746
Bertoni F, Bacchini P, Donati D, Martini A, Picci P, Campanacci M (1993) Osteoblastoma-like osteosarcoma. The Rizzoli Institute experience. Mod Pathol 6:707–716
El-Badawi ZH, Muhammad EM, Noaman HH (2012) Role of immunohistochemical cyclooxygenase-2 (COX-2) and osteocalcin in differentiating between osteoblastomas and osteosarcomas. Malays J Pathol 34:15–23
Riester SM, Torres-Mora J, Dudakovic A, Camilleri ET, Wang W, Xu F, Thaler RR, Evans JM, Zwartbol R, Briaire-de Bruijn IH, Maran A, Folpe AL, Inwards CY, Rose PS, Shives TC, Yaszemski MJ, Sim FH, Deyle DR, Larson AN, Galindo MA, Cleven AGH, Oliveira AM, Cleton-Jansen AM, Bovée JVMG, van Wijnen AJ (2017) Hypoxia-related microRNA-210 is a diagnostic marker for discriminating osteoblastoma and osteosarcoma. J Orthop Res 35:1137–1146. https://doi.org/10.1002/jor.23344
Baumhoer D, Amary F, Flanagan AM (2019) An update of molecular pathology of bone tumors. Lessons learned from investigating samples by next generation sequencing. Genes Chromosomes Cancer 58:88–99. https://doi.org/10.1002/gcc.22699
Fittall MW, Mifsud W, Pillay N, Ye H, Strobl AC, Verfaillie A, Demeulemeester J, Zhang L, Berisha F, Tarabichi M, Young MD, Miranda E, Tarpey PS, Tirabosco R, Amary F, Grigoriadis AE, Stratton MR, Van Loo P, Antonescu CR, Campbell PJ, Flanagan AM, Behjati S (2018) Recurrent rearrangements of FOS and FOSB define osteoblastoma. Nat Commun 9:2150. https://doi.org/10.1038/s41467-018-04530-z
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810. https://doi.org/10.1146/annurev.cellbio.20.010403.113126
Nord KH, Nilsson J, Arbajian E, Vult von Steyern F, Brosjö O, Cleton-Jansen AM, Szuhai K, Hogendoorn PC (2013) Recurrent chromosome 22 deletions in osteoblastoma affect inhibitors of the Wnt/beta-catenin signaling pathway. PLoS One 8:e80725. https://doi.org/10.1371/journal.pone.0080725
Wan Y, Zhao W, Jiang Y, Liu D, Meng G, Cai Y (2014) β-catenin is a valuable marker for differential diagnosis of osteoblastoma and osteosarcoma. Hum Pathol 45:1459–1465. https://doi.org/10.1016/j.humpath.2014.02.022
Ng TL, Gown AM, Barry TS, Cheang MC, Chan AK, Turbin DA, Hsu FD, West RB, Nielsen TO (2005) Nuclear beta-catenin in mesenchymal tumors. Mod Pathol 18:68–74. https://doi.org/10.1038/modpathol.3800272
Regard JB, Zhong Z, Williams BO, Yang Y (2012) Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harb Perspect Biol 4:a007997. https://doi.org/10.1101/cshperspect.a007997
Cai Y, Mohseny AB, Karperien M, Hogendoorn PC, Zhou G, Cleton-Jansen AM (2010) Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J Pathol 220:24–33. https://doi.org/10.1002/path.2628
Haydon RC, Deyrup A, Ishikawa A, Heck R, Jiang W, Zhou L, Feng T, King D, Cheng H, Breyer B, Peabody T, Simon MA, Montag AG, He TC (2002) Cytoplasmic and/or nuclear accumulation of the beta-catenin protein is a frequent event in human osteosarcoma. Int J Cancer 102:338–342. https://doi.org/10.1002/ijc.10719
Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M, Dobrovic A, Slavin J, Choong PF, Simmons PJ, Dawid IB, Thomas DM (2009) Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest 119:837–851. https://doi.org/10.1172/JCI37175
Chen K, Fallen S, Abaan HO, Hayran M, Gonzalez C, Wodajo F, MacDonald T, Toretsky JA, Uren A (2008) Wnt10b induces chemotaxis of osteosarcoma and correlates with reduced survival. Pediatr Blood Cancer 51:349–355. https://doi.org/10.1002/pbc.21595
Hoang BH, Kubo T, Healey JH, Sowers R, Mazza B, Yang R, Huvos AG, Meyers PA, Gorlick R (2004) Expression of LDL receptor-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. Int J Cancer 109:106–111. https://doi.org/10.1002/ijc.11677
Iwaya K, Ogawa H, Kuroda M, Izumi M, Ishida T, Mukai K (2003) Cytoplasmic and/or nuclear staining of beta-catenin is associated with lung metastasis. Clin Exp Metastasis 20:525–529. https://doi.org/10.1023/a:1025821229013
Iwao K, Miyoshi Y, Nawa G, Yoshikawa H, Ochi T, Nakamura Y (1999) Frequent beta-catenin abnormalities in bone and soft-tissue tumors. Jpn J Cancer Res 90:205–209. https://doi.org/10.1111/j.1349-7006.1999.tb00734.x
Amary MF, Berisha F, Mozela R, Gibbons R, Guttridge A, O’Donnell P, Baumhoer D, Tirabosco R, Flanagan AM (2016) The H3F3 K36M mutant antibody is a sensitive and specific marker for the diagnosis of chondroblastoma. Histopathology 69:121–127. https://doi.org/10.1111/his.12945
Behjati S, Tarpey PS, Presneau N, Scheipl S, Pillay N, van Loo P, Wedge DC, Cooke SL, Gundem G, Davies H, Nik-Zainal S, Martin S, McLaren S, Goody V, Robinson B, Butler A, Teague JW, Halai D, Khatri B, Myklebost O, Baumhoer D, Jundt G, Hamoudi R, Tirabosco R, Amary MF, Futreal PA, Stratton MR, Campbell PJ, Flanagan AM (2013) Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet 45:1479–1482. https://doi.org/10.1038/ng.2814
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Kivilcim Eren Erdogan, Marina Pacheco, Marco Gambarotti, Giovanna Magagnoli, Marta Sbaraglia, Tommaso Frisoni, and Alberto Righi. The draft of the manuscript was written by Kivilcim Eren Erdogan, Marina Pacheco, Alberto Righi, and Angelo Paolo Dei Tos. All authors read and approved the final manuscript.
Funding
Fundación Martha Stella C. de Vallarino for financial support through a medical educational scholarship to one of the authors (M. Pacheco).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Erdogan, K.E., Pacheco, M., Gambarotti, M. et al. Usefulness of β-catenin expression in the differential diagnosis of osteosarcoma, osteoblastoma, and chondroblastoma. Virchows Arch 479, 529–535 (2021). https://doi.org/10.1007/s00428-020-03004-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-03004-2