Abstract
Salivary duct carcinoma (SDC) is an aggressive, uncommon tumor histologically comparable to high-grade mammary ductal carcinoma. SDCs are usually androgen receptor (AR)–positive and often HER2-positive. Recently, therapies targeting these molecules for SDC have attracted attention. Lipid metabolism changes have been described in association with biological behavior in various cancers, although no such relationship has yet been reported for SDC. We therefore analyzed the clinicopathological relevance of the immunohistochemical expression of adipophilin (ADP) and fatty acid synthase (FASN), representative lipid metabolism–related proteins, in 147 SDCs. ADP and FASN were variably immunoreactive in most SDCs (both 99.3%), and the ADP and FASN expression was negatively correlated (P = 0.014). ADP-positive (≥ 5%) SDCs more frequently exhibited a prominent nuclear pleomorphism and high-Ki-67 labeling index than those ADP-negative (P = 0.013 and 0.011, respectively). In contrast, a high FASN score, calculated by the staining proportion and intensity, (≥ 120) was correlated with the high expression of AR and FOXA1 (P < 0.001 and = 0.003, respectively). The ADP and FASN expression differed significantly among the subtypes based on biomarker immunoprofiling, as assessed by the AR, HER2, and Ki-67 status (P = 0.017 and 0.003, respectively). A multivariate analysis showed that ADP-positive expression was associated with a shorter overall and progression-free survival (P = 0.018 and 0.003, respectively). ADP was associated with an aggressive histopathology and unfavorable prognosis, and FASN may biologically interact with the AR signaling pathway in SDC. ADP may, therefore, be a new prognostic indicator and therapeutic target in SDC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salivary duct carcinoma (SDC) is an aggressive and uncommon tumor that accounts for as many as 10% of all salivary gland malignancies. It can occur not only as de novo carcinoma but also as a malignant component of carcinoma ex pleomorphic adenoma [1]. SDC is histologically comparable to high-grade mammary ductal carcinoma and recent transcriptome data have also shown striking similarities between SDC and apocrine breast cancer [2]. Most SDCs express androgen receptor (AR) and its related protein, FOXA1, but not estrogen receptor (ER) or progesterone receptor, and approximately 40% are positive for HER2 [3, 4].
While normal cells produce energy by aerobic phosphorylation through the tricarboxylic acid cycle, cancer cells produce energy by aerobic glycolysis and other metabolic pathways. The oncogenic metabolic pathways differ depending on the tumor types and, therefore, the development of therapies against tumor metabolism is not straightforward [5]. Lipid metabolism is a crucial pathway in tumor progression and cancer cells typically show lipid accumulation [6, 7].
Adipophilin (ADP), also known as perilipin 2 or adipose differentiation–related protein, covers intracytoplasmic lipid droplets. ADP prevents the efflux of intracellular lipid droplets and increases the intracellular lipid level [8]. Immunohistochemically, ADP is a diagnostic marker of apocrine differentiation in breast cancer, and lipid-storage cancers such as sebaceous carcinoma and secretory carcinoma of the salivary glands [9,10,11,12]. Furthermore, the ADP expression was recently found to be a poor prognosticator in various malignant tumors, including pulmonary, pancreatic, colonic, and renal cell carcinomas, and Burkitt lymphoma [13,14,15,16,17]. In breast cancer, ADP is related to the ER expression, HER2 status, and the classification based on the biomarker immunoprofiling [9].
Fatty acid synthase (FASN) is a key lipogenic enzyme and is also overexpressed in various human cancers including salivary tumors [18, 19]. FASN expression has been reported to be associated with a poor prognosis in several types of tumors [20, 21]. In addition, in breast cancer, like ADP, the FASN expression is correlated with the HER2 status and the subtype of the immunoprofiling classification, and in prostate cancer, the FASN expression is correlated with the AR expression [22,23,24].
To our knowledge, the role of lipid metabolism–related proteins in SDCs has not yet been described. In the present study, we immunohistochemically evaluated the ADP and FASN expression and investigated their clinicopathological significance in a series of 147 SDCs.
Materials and methods
This study was approved by the Institutional Ethics Review Board of each participating institution.
Patients
All patients underwent a central pathological review by an expert pathologist (T.N.) according to the rigorous histomorphologic criteria for SDC. We recruited 147 patients who were diagnosed with and received treatment for SDC at seven institutions between 1992 and 2014. Patients who were treated with anti-AR or anti-HER2 therapy as a first-line treatment were excluded from this study. We retrospectively reviewed the patient records to obtain information about the age, sex, tumor size, lymph node metastasis, other distant metastasis, and outcome. Tumor staging was classified in accordance with the seventh ed. TNM classification of the International Union Against Cancer 2009.
Histopathology
The histopathological analysis of nuclear pleomorphism was based on our previous report [25]. In brief, we evaluated the presence of marked variation in the size of tumor cell nuclei throughout the tumor, rather than focal, regardless of the absolute nuclear size.
Immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH)
For IHC, formalin-fixed, paraffin-embedded tumor tissue was cut into 4-μm-thick sections. A polymer-based detection system with heat-mediated antigen retrieval was conducted using the primary antibodies shown in Supplementary Table 1. Diaminobenzidine was applied to detect antigen-antibody reactions. The ADP expression was considered to be positive when the globular or granular cytoplasmic expression was found in ≥ 5% of the tumor cells, as previously reported [13, 15]. FASN was analyzed using a combined scoring system based on both the proportion of positive tumor cells (0–100%) and the predominant staining intensity in the tumor [26]. The FASN staining intensity was scored categorically (0, negative; 1, weak; 2, moderate; 3, strong). FASN score (0–300) was calculated by multiplying the percentage by the staining intensity. SDC cases were classified into two groups based on FASN score: low (< 120) and high (≥ 120). A case was considered high for AR and FOXA1 when ≥ 20% of the tumor cell nuclei showed strong staining [4, 27]. HER2 was considered to be positive based on an HER2 IHC score of 3+ and/or HER2 amplification, as determined by a FISH analysis, in accordance with the ASCO/CAP guideline for evaluating breast cancer [28]. The results of p53 staining were interpreted based on the expression pattern. Cases were classified into three groups as follows: extreme negative, complete confluent negativity of staining; extreme positive, strong diffuse confluent positivity; and non-extreme, all intermediate expression of any intensity [27]. The percentage of Ki-67-positive cells was determined by counting at least 1000 tumor cells, and then recorded as the Ki-67 labeling index (LI). Ki-67 LI values of < 40% and ≥ 40% were classified as Ki-67-low and Ki-67-high, respectively [9]. The HER2 status and the results of immunohistochemical staining of AR, FOXA1, Ki-67, and p53, which were previously reported by our group, were compared to the ADP and FASN expression [4, 27, 29].
Classification based on the biomarker immunoprofile
The classifications of SDC were based on our previous description. All SDCs were categorized into five main subtypes based on a combination of the expression of AR (instead of ER or PR for breast cancer), HER2 (or the HER2 amplification status), and Ki-67 as follows: “apocrine A” (AR+/HER2−/Ki-67-low), “apocrine B” (AR+/HER2−/Ki-67-high), “apocrine HER2” (AR+/HER2+), “HER2-enriched” (AR−/HER2+), and “double-negative” (AR−/HER2−) [27].
Statistical analyses
Non-continuous variables were compared using the chi-squared test. Continuous variables were compared using the Mann-Whitney U test. Spearman’s rank correlation test was used to evaluate the association between the protein expression. The association between the ADP expression and overall survival (OS) or progression-free survival (PFS) was evaluated using the Kaplan-Meier product-limit method and univariate and multivariate Cox proportional hazard models. The potential confounders in the multivariate analysis included the age (< 65 vs. ≥ 65 years), sex (male vs. female), primary tumor site (parotid gland vs. submandibular gland vs. other), T classification (1–4), N classification (0 vs. 1, 2), M classification (0, 1), first-line treatment (surgery vs. other), and histological type (de novo vs. carcinoma ex pleomorphic adenoma). All statistical tests were performed using the STATA software program (version 13, StataCorp, College Station, TX, USA). All tests were two-sided, and P values of < 0.05 were considered to indicate statistical significance.
Results
Histological features and patient characteristics
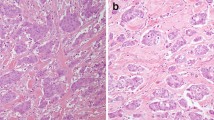
Representative histological findings in a case of SDC are shown in Fig. 1. All cases of SDC had abundant granular and eosinophilic cytoplasm and sometimes displayed an apocrine snout-like morphology (i.e., apocrine-like features). Histopathological variants of SDC were identified as sarcomatoid (n = 9; 6.1%), invasive micropapillary (n = 6; 4.1%), and mucin-rich (n = 2; 1.4%) variants. The patient characteristics are shown in Table 1. Prominent nuclear pleomorphism was observed in 97 cases (66%) (Figs. 1c, d). The median follow-up period of the survivors was 3.4 years (range, 0.04–19.0 years). The 3-year OS rate in all patients was 69.0% (95% confidence interval [CI], 60.5%–76.0%), while the 3-year PFS rate was 37.5% (95% CI, 29.4%–45.6%).
Representative histologic features of salivary duct carcinoma (SDC). a SDC consisted of intraductal and invasive components. b Carcinoma cells have abundant, granular, and eosinophilic cytoplasm and display an apocrine snout-like morphology (i.e., apocrine-like features). c An example of a SDC case with prominent nuclear pleomorphism. d An example of a SDC case without prominent nuclear pleomorphism
The expression of ADP and FASN
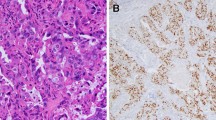
Among the 147 SDC tumor specimens, ADP and FASN were expressed in at least a limited part of the tumor in 146 cases (both in 99.3%). The ADP expression of foamy macrophages in the tubular lumen (Fig. 2a) and the FASN expression of the adipose tissue were used as an internal positive control [9, 10]. Thirty-five cases (23.8%) and 112 cases (76.2%) were classified into the ADP-negative and ADP-positive groups, respectively (mean ADP expression value 13.5%) (Figs. 2a, b), and 30 cases (20.6%) and 116 cases (79.4%) were categorized into the FASN-low and FASN-high groups, respectively (mean FASN score 168.1) (Figs. 2c, d).
The adipophilin expression is immunohistochemically detected in a 0% and, b 100% of salivary duct carcinomas. Note that foamy macrophages in the tubular lumen were used as a built-in positive control (arrow) (a). The fatty acid synthase score is immunohistochemically c low (80) and d high (300) in salivary duct carcinomas
The correlations between ADP/FASN expression and the clinicopathological factors, and various biomarkers are summarized in Table 1. A negative correlation was observed between the expression of ADP and FASN (P = 0.014). An ADP-positive tumor was associated with the presence of a prominent nuclear pleomorphism and a high Ki-67 labeling index (P = 0.013 and 0.011, respectively). A marginally significant association was detected between ADP and HER2 positivity (P = 0.058). A FASN-high status was associated with high AR and FOXA1 expression levels (P < 0.001, and 0.003, respectively). A Spearman’s rank correlation test revealed that the FASN score was correlated significantly and positively with the AR and FOXA1 expression (r = 0.315, P < 0.001 and r = 0.277, P < 0.001, respectively). No variables were correlated with sex, age, TNM classification, or histological origin. The ADP and FASN expression levels differed significantly among the different biomarker immunoprofile subtypes (P = 0.017 and 0.003, respectively) (Supplementary Table 2). The ratio of cases with ADP-positive was highest in the “HER2-enriched” group and lowest in the “Apocrine A” group, whereas the ratio of cases with a high-FASN expression was highest in the “Apocrine B” group and lowest in the “HER2-enriched” group.
Prognostic impact
The results of the univariate and multivariate analyses are shown in Table 2. The univariate and multivariate analyses revealed that ADP-positive expression was significantly associated with shorter OS and PFS. The Kaplan-Meier survival curves of the association between the ADP expression and the clinical outcomes are shown in Fig. 3. However, a significant association between the FASN expression and the PFS was found only in the multivariate analysis, not in the univariate analysis. There was no significant association between the FASN expression and the OS.
Discussion
In the present study, we analyzed the lipid metabolism–related protein expression, focusing on ADP and FASN, for their clinicopathological relevance in SDC. We demonstrated that almost all of the 147 SDC cases expressed ADP and FASN, with a diverse ratio and intensity of positive cells, and that there was an association between ADP and FASN expression. ADP-positive expression was associated with the presence of a prominent nuclear pleomorphism (i.e., histological aggressiveness), high Ki-67 labeling index, and a poor prognosis. Furthermore, the high expression of FASN was positively correlated with AR and FOXA1 expression. The ADP and FASN status was also linked with the biomarker-based classification of SDC.
To our knowledge, this is the first study to validate ADP as an independent unfavorable prognostic factor in a large cohort of SDC patients. The following three hypotheses might support the results. First, the high expression of ADP is associated with histological aggressiveness and consequently results in a poor prognosis. Lung and pancreatic cancers with high ADP expression often show high-grade morphological features and poor prognosis [13, 15]. We found a similar relationship in SDC. Second, in SDC, intracellular lipids may stimulate tumor cell proliferation and this could result in an unfavorable prognosis. In a colon carcinoma cell line, the accumulated lipid droplets promoted cellular proliferation, whereas the silencing of ADP inhibited cellular proliferation [30]. In this study, ADP-positive expression was related to a high Ki-67 labeling index, a representative marker of cellular proliferation. This relationship was also reported in lung and breast cancer [9, 13]. Third, in SDC, the expression of ADP may reflect the activation of hypoxic signaling, and this activation may lead to an aggressive phenotype and a poor prognosis. The accumulation of intracellular lipid droplets is a usual observation in ischemic tissues, such as organ infarction [7]. In cancer, hypoxic signaling usually is activated by ischemia and is related to an aggressive phenotype [7, 31]. It is suggested that the coordination of the metabolic deregulation and hypoxic signaling contributed to the biological aggressiveness of lung adenocarcinoma [31]. To verify these three hypotheses, further studies of the functional role of ADP in SDC is required.
In SDC, the FASN score was correlated significantly and positively with the AR and FOXA1 expression. In prostate cancer, selective FASN inhibition antagonizes tumor growth through metabolic reprogramming and results in a reduced protein expression and reduced transcriptional activity of AR. Activation of the endoplasmic reticulum stress response, resulting in reduced protein synthesis, was involved in the FASN inhibition of the AR pathway [24]. In SDC, as well as prostate cancer, the expression of FASN might be associated with the expression of AR through the endoplasmic reticulum stress response. The association between the expression of FASN and FOXA1 in cancer has not been examined. In SDC, the FOXA1 expression was positively correlated with the expression of AR [4]; thus, there might be a positive relationship between FASN and FOXA1. Recently, therapies targeting AR have been shown to be effective in SDC patients and have attracted attention [32]. The overexpression of FASN was reported to be linked to castration-resistant prostate cancer growth and resistance to chemotherapy [24, 33]. Future studies are expected to clarify the association between these therapies and the FASN expression in SDC.
In this study, a negative correlation was observed between the expression of ADP and FASN in SDC. Both positive and negative correlations have been reported in previous studies in different types of cells [34, 35]. ADP reflects the presence of intracellular lipid accumulation well [9, 36]. FASN is known to be a central enzyme in de novo lipogenesis primarily from carbohydrate sources, inducing lipid accumulation [37]. However, lipid accumulation in cells is derived from not only de novo lipogenesis but also the uptake of lipids and neutral lipid synthesis, irrespective of FASN [35]. Further studies will be necessary to clarify these mechanisms and their significance in SDC.
In breast cancer, ADP and FASN have been reported to be associated with the immune-biomarker classification, which is a surrogate for molecular subtyping reflecting the different metabolic pathways [9, 22]. Given that FASN inhibitors have shown cytotoxicity in various cancers [18, 38], the different expressions of ADP and FASN in accordance with the SDC subtypes assessed by the AR (instead of ER or PR for breast cancer), HER2, and Ki-67 status might imply that inhibiting proteins related to lipid metabolism is a possible therapeutic approach for some subtypes. Although we failed to prove the influence of the FASN expression in SDC on the prognosis, patients with a high FASN expression score may be suitable for FASN inhibitor therapy in the future, regardless of the prognostic significance. However, further analyses are needed to establish whether or not lipid metabolism–related proteins are suitable therapeutic targets in SDC.
In conclusion, this study showed that ADP and FASN are frequently but unevenly expressed in SDC. ADP is related to histological aggressiveness and has prognostic significance with an unfavorable survival outcome in SDC patients. ADP may, therefore, be a new prognostic indicator and a novel therapeutic target associated with lipid metabolism. In addition, FASN might biologically interact with the AR signaling pathway.
References
Nagao T, Licitra L, Loening T, Vielh P, Williams M (2017) Salivary duct carcinoma. In: El-Naggar A, Chan J, Grandis J, Takata T, Slootweg P (eds) World Health Organization classification of tumours, 4th edn. IARC, Lyon, pp 173–174
Dalin MG, Desrichard A, Katabi N, Makarov V, Walsh LA, Lee KW, Wang Q, Armenia J, West L, Dogan S, Wang L, Ramaswami D, Ho AL, Ganly I, Solit DB, Berger MF, Schultz ND, Reis-Filho JS, Chan TA, Morris LG (2016) Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin Cancer Res 22:4623–4633
Masubuchi T, Tada Y, Maruya S, Osamura Y, Kamata SE, Miura K, Fushimi C, Takahashi H, Kawakita D, Kishimoto S, Nagao T (2015) Clinicopathological significance of androgen receptor, HER2, Ki-67 and EGFR expressions in salivary duct carcinoma. Int J Clin Oncol 20:35–44
Urano M, Hirai H, Tada Y, Kawakita D, Shimura T, Tsukahara K et al (2018) The high expression of FOXA1 is correlated with a favourable prognosis in salivary duct carcinomas: a study of 142 cases. Histopathology 73:943–952
Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E (2007) Energy metabolism in tumor cells. FEBS J 274:1393–1418
Porporato PE, Payen VL, Baselet B, Sonveaux P (2016) Metabolic changes associated with tumor metastasis, part 2: mitochondria, lipid and amino acid metabolism. Cell Mol Life Sci 73:1349–1363
Straub BK, Gyoengyoesi B, Koenig M, Hashani M, Pawella LM, Herpel E, Mueller W, Macher-Goeppinger S, Heid H, Schirmacher P (2013) Adipophilin/perilipin-2 as a lipid droplet-specific marker for metabolically active cells and diseases associated with metabolic dysregulation. Histopathology 62:617–631
Larigauderie G, Furman C, Jaye M, Lasselin C, Copin C, Fruchart JC et al (2004) Adipophilin enhances lipid accumulation and prevents lipid efflux from THP-1 macrophages: potential role in atherogenesis. Arterioscler Thromb Vasc Biol 24:504–510
Kuniyoshi S, Miki Y, Sasaki A, Iwabuchi E, Ono K, Onodera Y et al (2019) The significance of lipid accumulation in breast carcinoma cells through perilipin 2 and its clinicopathological significance. Pathol Int 69:463–471
Moritani S, Ichihara S, Hasegawa M, Endo T, Oiwa M, Shiraiwa M, Nishida C, Morita T, Sato Y, Hayashi T, Kato A (2011) Intracytoplasmic lipid accumulation in apocrine carcinoma of the breast evaluated with adipophilin immunoreactivity: a possible link between apocrine carcinoma and lipid-rich carcinoma. Am J Surg Pathol 35:861–867
Ostler DA, Prieto VG, Reed JA, Deavers MT, Lazar AJ, Ivan D (2010) Adipophilin expression in sebaceous tumors and other cutaneous lesions with clear cell histology: an immunohistochemical study of 117 cases. Mod Pathol 23:567–573
Urano M, Nagao T, Miyabe S, Ishibashi K, Higuchi K, Kuroda M (2015) Characterization of mammary analogue secretory carcinoma of the salivary gland: discrimination from its mimics by the presence of the ETV6-NTRK3 translocation and novel surrogate markers. Hum Pathol 46:94–103
Fujimoto M, Yoshizawa A, Sumiyoshi S, Sonobe M, Menju T, Hirata M, Momose M, Date H, Haga H (2017) Adipophilin expression in lung adenocarcinoma is associated with apocrine-like features and poor clinical prognosis: an immunohistochemical study of 328 cases. Histopathology 70:232–241
Ambrosio MR, Piccaluga PP, Ponzoni M, Rocca BJ, Malagnino V, Onorati M et al (2012) The alteration of lipid metabolism in Burkitt lymphoma identifies a novel marker: adipophilin. PLoS One 7:e44315
Hashimoto Y, Ishida M, Ryota H, Yamamoto T, Kosaka H, Hirooka S, Yamaki S, Kotsuka M, Matsui Y, Yanagimoto H, Tsuta K, Satoi S (2019) Adipophilin expression is an indicator of poor prognosis in patients with pancreatic ductal adenocarcinoma: an immunohistochemical analysis. Pancreatology 19:443–448
Matsubara J, Honda K, Ono M, Sekine S, Tanaka Y, Kobayashi M et al (2011) Identification of adipophilin as a potential plasma biomarker for colorectal cancer using label-free quantitative mass spectrometry and protein microarray. Cancer Epidemiol Biomark Prev 20:2195–2203
Yao M, Huang Y, Shioi K, Hattori K, Murakami T, Nakaigawa N, Kishida T, Nagashima Y, Kubota Y (2007) Expression of adipose differentiation-related protein: a predictor of cancer-specific survival in clear cell renal carcinoma. Clin Cancer Res 13:152–160
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7:763–777
Díaz KP, Gondak R, Martins LL, de Almeida OP, León JE, Mariano FV, Altemani A, Vargas PA (2019) Fatty acid synthase and Ki-67 immunoexpression can be useful for the identification of malignant component in carcinoma ex-pleomorphic adenoma. J Oral Pathol Med 48:232–238
Ogino S, Nosho K, Meyerhardt JA, Kirkner GJ, Chan AT, Kawasaki T, Giovannucci EL, Loda M, Fuchs CS (2008) Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol 26:5713–5720
Abdelrahman AE, Rashed HE, Elkady E, Elsebai EA, El-Azony A, Matar I (2019) Fatty acid synthase, Her2/neu, and E2F1 as prognostic markers of progression in non-muscle invasive bladder cancer. Ann Diagn Pathol 39:42–52
Kim S, Lee Y, Koo JS (2015) Differential expression of lipid metabolism-related proteins in different breast cancer subtypes. PLoS One 10:e0119473
Corominas-Faja B, Vellon L, Cuyàs E, Buxó M, Martin-Castillo B, Serra D, García J, Lupu R, Menendez JA (2017) Clinical and therapeutic relevance of the metabolic oncogene fatty acid synthase in HER2+ breast cancer. Histol Histopathol 32:687–698
Zadra G, Ribeiro CF, Chetta P, Ho Y, Cacciatore S, Gao X, Syamala S, Bango C, Photopoulos C, Huang Y, Tyekucheva S, Bastos DC, Tchaicha J, Lawney B, Uo T, D'Anello L, Csibi A, Kalekar R, Larimer B, Ellis L, Butler LM, Morrissey C, McGovern K, Palombella VJ, Kutok JL, Mahmood U, Bosari S, Adams J, Peluso S, Dehm SM, Plymate SR, Loda M (2019) Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc Natl Acad Sci U S A 116:631–640
Nakaguro M, Sato Y, Tada Y, Kawakita D, Hirai H, Urano M et al (2019) Prognostic implication of histopathologic indicators in salivary duct carcinoma: proposal of a novel histologic risk stratification model. Am J Surg Pathol. https://doi.org/10.1097/PAS.0000000000001413
de Andrade BA, Leon JE, Carlos R, Delgado-Azanero W, Mosqueda-Taylor A, Graner E et al (2011) Expression of fatty acid synthase (FASN) in oral nevi and melanoma. Oral Dis 17:808–812
Takase S, Kano S, Tada Y, Kawakita D, Shimura T, Hirai H, Tsukahara K, Shimizu A, Imanishi Y, Ozawa H, Okami K, Sato Y, Sato Y, Fushimi C, Okada T, Sato H, Otsuka K, Watanabe Y, Sakai A, Ebisumoto K, Togashi T, Ueki Y, Ota H, Hanazawa T, Chazono H, Osamura RY, Nagao T (2017) Biomarker immunoprofile in salivary duct carcinomas: clinicopathological and prognostic implications with evaluation of the revised classification. Oncotarget 8:59023–59035
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical Oncology, College of American Pathologists (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013
Shimura T, Tada Y, Hirai H, Kawakita D, Kano S, Tsukahara K, Shimizu A, Takase S, Imanishi Y, Ozawa H, Okami K, Sato Y, Sato Y, Fushimi C, Takahashi H, Okada T, Sato H, Otsuka K, Watanabe Y, Sakai A, Ebisumoto K, Togashi T, Ueki Y, Ota H, Ando M, Kohsaka S, Hanazawa T, Chazono H, Kadokura Y, Kobayashi H, Nagao T (2018) Prognostic and histogenetic roles of gene alteration and the expression of key potentially actionable targets in salivary duct carcinomas. Oncotarget 9:1852–1867
Qi W, Fitchev PS, Cornwell ML, Greenberg J, Cabe M, Weber CR et al (2013) FOXO3 growth inhibition of colonic cells is dependent on intraepithelial lipid droplet density. J Biol Chem 288:16274–16281
Shin SA, Na HY, Choe JY, Chung D, Park M, Oh S, Kim JE (2018) The expression of adipophilin is frequently found in solid subtype adenocarcinoma and is associated with adverse outcomes in lung adenocarcinoma. J Pathol Transl Med 52:357–362
Fushimi C, Tada Y, Takahashi H, Nagao T, Ojiri H, Masubuchi T et al (2018) A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol 29:979–984
Wu X, Dong Z, Wang CJ, Barlow LJ, Fako V, Serrano MA, Zou Y, Liu JY, Zhang JT (2016) FASN regulates cellular response to genotoxic treatments by increasing PARP-1 expression and DNA repair activity via NF-kappaB and SP1. Proc Natl Acad Sci U S A 113:E6965–E6973
Faleck DM, Ali K, Roat R, Graham MJ, Crooke RM, Battisti R, Garcia E, Ahima RS, Imai Y (2010) Adipose differentiation-related protein regulates lipids and insulin in pancreatic islets. Am J Physiol Endocrinol Metab 299:E249–E257
Chang R, Chou MC, Hung LY, Wang ME, Hsu MC, Chiu CH (2016) Study of valproic acid-enhanced hepatocyte steatosis. Biomed Res Int. https://doi.org/10.1155/2016/9576503
Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C (1997) Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38:2249–2263
Wang Y, Jones Voy B, Urs S, Kim S, Soltani-Bejnood M, Quigley N, Heo YR, Standridge M, Andersen B, Dhar M, Joshi R, Wortman P, Taylor JW, Chun J, Leuze M, Claycombe K, Saxton AM, Moustaid-Moussa N (2004) The human fatty acid synthase gene and de novo lipogenesis are coordinately regulated in human adipose tissue. J Nutr 134:1032–1038
Lupien LE, Dunkley EM, Maloy MJ, Lehner IB, Foisey MG, Ouellette ME, Lewis LD, Pooler DB, Kinlaw WB, Baures PW (2019) An inhibitor of fatty acid synthase thioesterase domain with improved cytotoxicity against breast cancer cells and stability in plasma. J Pharmacol Exp Ther 371:171–185
Acknowledgments
The authors thank Mayumi Yokotsuka for performing the gene mutation analysis and Hitomi Yokota and Yoshinari Yamamoto for performing the immunohistochemical analysis.
Funding
This work was supported by a Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (C) to Yuichiro Tada (No. 18K09836) and Toshitaka Nagao (No. 17K08705), and a JSPS Grant-in-Aid for Young Scientists (B) to Hideaki Hirai (No. 16K19072) and Daisuke Kawakita (No. 17K18006).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, study design, material preparation, and data collection. Data analysis was performed by Hideaki Hirai, Yuichiro Tada, Masato Nakaguro, Daisuke Kawakita, and Toshitaka Nagao. The first draft of the manuscript was written by Hideaki Hirai, Yuichiro Tada, and Masato Nakaguro, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
This study was performed according to the Declaration of Helsinki. The study protocol was reviewed and approved by the Institutional Review Board of the International University of Health and Welfare Mita Hospital (No. 5-19-58).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Quality in Pathology
Electronic supplementary material
ESM 1
(XLSX 15 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hirai, H., Tada, Y., Nakaguro, M. et al. The clinicopathological significance of the adipophilin and fatty acid synthase expression in salivary duct carcinoma. Virchows Arch 477, 291–299 (2020). https://doi.org/10.1007/s00428-020-02777-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02777-w