Abstract
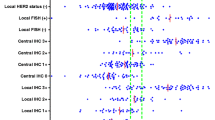
To compare results from messenger RNA (mRNA)-based TargetPrint testing with those from immunohistochemistry (IHC) and in situ hybridization (ISH) conducted according to local standard procedures at hospitals worldwide. Tumor samples were prospectively obtained from 806 patients at 22 hospitals. The mRNA level of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) was assessed by TargetPrint quantitative gene expression readouts. IHC/ISH assessments were performed according to local standards at the participating hospitals. TargetPrint readout showed a high concordance with IHC/ISH of 95 % (kappa 0.81) for ER, 81 % (kappa 0.56) for PR, and 94 % (kappa 0.76) for HER2. The positive/negative agreement between TargetPrint and IHC for ER, PR, and HER2 was 96 %/87 %, 84 %/74 %, and 74 %/98 %, respectively. The concordance rate in IHC/ISH results between hospitals varied: 88–100 % for ER (kappa 0.50–1.00); 50–100 % for PR (kappa 0.20–1.00); and 90–100 % for HER2 (kappa 0.59–1.00). mRNA readout of ER, PR, and HER2 status by TargetPrint was largely comparable to local IHC/ISH analysis. However, there was substantial discordance in IHC/ISH results between different hospitals. When results are discordant, the use of TargetPrint would improve the reliability of hormone receptor and HER2 results by prompting retesting in a reference laboratory.


Similar content being viewed by others
References
Febbo PG, Ladanyi M, Aldape KD, et al: NCCN task force report: evaluating the clinical utility of tumor markers in oncology. J Natl Compr Canc Netw 2011;(suppl 5):S1-S32
Liu S, Chia SK, Mehl E, et al. (2010) Progesterone receptor is a significant factor associated with clinical outcomes and effect of adjuvant tamoxifen therapy in breast cancer patients. Breast Cancer Res Treat 119:53–61
Loi S, Haibe-Kains B, Desmedt C, et al. (2008) Predicting prognosis using molecular profiling in estrogen receptor-positive breast cancer treated with tamoxifen. BMC Genomics 9:239
Goldhirsch A, Ingle JN, Gelber RD, et al. (2009) Thresholds for therapies: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20:1319–1329
Goldhirsch A, Wood WC, Coates AS, et al. (2011) Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22:1736–1747
Harris L, Fritsche H, Mennel R, et al. (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25:5287–5312
National Comprehensive Cancer Network: NCCN 2015 Clinical practice guidelines in oncology. Breast Cancer. Version 2.2015.http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
Bordeaux JM, Cheng H, Welsh AW, et al. (2012) Quantitative in situ measurement of estrogen receptor mRNA predicts response to tamoxifen. PLoS One 7:e36559
Iverson AA, Gillett C, Cane P, et al. (2009) A single-tube quantitative assay for mRNA levels of hormonal and growth factor receptors in breast cancer specimens. J Mol Diagn 11:117–130
Nguyen B, Cusumano PG, Deck K, et al. (2012) Comparison of molecular subtyping with BluePrint, MammaPrint, and TargetPrint to local clinical subtyping in breast cancer patients. Ann Surg Oncol 19:3257–3263
Roepman P, Horlings HM, Krijgsman O, et al. (2009) Microarray-based determination of estrogen receptor, progesterone receptor, and HER2 receptor status in breast cancer. Clin Cancer Res 15:7003–7011
Viale G, Slaets L, Bogaerts J, et al. (2014) High concordance of protein (by IHC), gene (by FISH; HER2 only) and microarray readout (by TargetPrint) of ER/PR/HER2: results from EORTC 10041/BIG 03-04 MINDACT trial. Ann Oncol 25(4):816–823
Allred DC (2010) Issues and updates: evaluating estrogen receptor-alpha, progesterone receptor, and HER2 in breast cancer. Mod Pathol 23(suppl 2):S52–S59
Arihiro K, Umemura S, Kurosumi M, et al. (2007) Comparison of evaluations for hormone receptors in breast carcinoma using two manual and three automated immunohistochemical assays. Am J Clin Pathol 127:356–365
Oyama T, Ishikawa Y, Hayashi M, et al. (2007) The effects of fixation, processing and evaluation criteria on immunohistochemical detection of hormone receptors in breast cancer. Breast Cancer 14:182–188
Bueno-de-Mesquita JM, Nuyten DS, Wesseling J, et al. (2010) The impact of inter-observer variation in pathological assessment of node-negative breast cancer on clinical risk assessment and patient selection for adjuvant systemic treatment. Ann Oncol 21:40–47
Hammond ME, Hayes DF, Dowsett M, et al. (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795
Carlson RW, Moench SJ, Hammond ME, et al. (2006) HER2 testing in breast cancer: NCCN task force report and recommendations. J Natl Compr Cancer Netw 4(suppl 3):S1–S22
Wolff AC, Hammond ME, Schwartz JN, et al. (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Fitzgibbons PL, Murphy DA, Hammond ME, et al. (2010) Recommendations for validating estrogen and progesterone receptor immunohistochemistry assays. Arch Pathol Lab Med 134:930–935
Allred DC (2008) Commentary: hormone receptor testing in breast cancer: a distress signal from Canada. Oncologist 13:1134–1136
Hede K (2008) Breast cancer testing scandal shines spotlight on black box of clinical laboratory testing. J Natl Cancer Inst 100:836–837
Rhodes A, Jasani B, Barnes DM, et al. (2000) Reliability of immunohistochemical demonstration of oestrogen receptors in routine practice: interlaboratory variance in the sensitivity of detection and evaluation of scoring systems. J Clin Pathol 53:125–130
Viale G, Regan MM, Maiorano E, et al. (2007) Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J Clin Oncol 25:3846–3852
Perez EA, Suman VJ, Davidson NE, et al. (2006) HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol 24:3032–3038
Reddy JC, Reimann JD, Anderson SM, et al. (2006) Concordance between central and local laboratory HER2 testing from a community-based clinical study. Clin Breast Cancer 7:153–157
Chang HR (2010) Trastuzumab-based neoadjuvant therapy in patients with HER2-positive breast cancer. Cancer 116:2856–2867
Hortobagyi GN (2012) Toward individualized breast cancer therapy: translating biological concepts to the bedside. Oncologist 17:577–584
Delahaye L, Wehkamp D, Floore A, et al. (2013) Analytical validity of the MammaPrint® breast cancer diagnostic gene signature. Perinat Med 10(8):801–811
Roepman P, Schuurman A, Delahaye LJ, et al: A gene expression profile for detection of sufficient tumour cells in breast tumour tissue: microarray diagnosis eligibility. BMC Med Genet 2009 Aug 12;2:52.
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20:37–46
Viale G, Slaets L, de Snoo FA, et al. Discordant assessment of tumor biomarkers by histopathological and molecular assays in the EORTC randomized controlled 10041/BIG 03-04 MINDACT trial breast cancer : intratumoral heterogeneity and DCIS or normal tissue components are unlikely to be the cause of discordance. Breast Cancer Res Treat 2016 Feb. 155(3):463–469
Ma XJ, Hilsenbeck SG, Wang W, et al. (2006) The HOXB13:IL17BR expression index is a prognostic factor in early-stage breast cancer. J Clin Oncol 24:4611–4619
TO N, Parker JS, Leung S, et al. (2010) A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 16:5222–5232
Umemura S, Osamura RY, Akiyama F, et al. (2008) What causes discrepancies in HER2 testing for breast cancer? A Japanese ring study in conjunction with the global standard. Am J Clin Pathol 130:883–891
Tsuda H, Kurosumi M, Umemura S, et al. (2010) HER2 testing on core needle biopsy specimens from primary breast cancers: interobserver reproducibility and concordance with surgically resected specimens. BMC Cancer 10:534
Baehner FL, Achacoso N, Maddala T, et al. (2010) Human epidermal growth factor receptor 2 assessment in a case-control study: comparison of fluorescence in situ hybridization and quantitative reverse transcription polymerase chain reaction performed by central laboratories. J Clin Oncol 28:4300–4306
Dabbs DJ, Klein ME, Mohsin SK, et al. (2011) High false-negative rate of HER2 quantitative reverse transcription polymerase chain reaction of the Oncotype DX test: an independent quality assurance study. J Clin Oncol 29:4279–4285
Pinhel I, Hills M, Drury S, et al. (2012) ER and HER2 expression are positively correlated in HER2 non-overexpressing breast cancer. Breast Cancer Res 14:R46
Dowsett M, Hanna WM, Kockx M, et al. (2007) Standardization of HER2 testing: results of an international proficiency-testing ring study. Mod Pathol 20:584–591
McCullough AE, Dell'orto P, Reinholz MM, et al. Central pathology laboratory review of HER2 and ER in early breast cancer: an ALTTO trial [BIG 2-06/NCCTG N063D (alliance)] ring study. Breast Cancer Res Treat 2014 Feb. 143(3):485–492.
Dowsett M, Allred C, Knox J, et al. (2008) Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the arimidex, tamoxifen, alone or in combination trial. J Clin Oncol 26:1059–1065
Phillips T, Murray G, Wakamiya K, et al. (2007) Development of standard estrogen and progesterone receptor immunohistochemical assays for selection of patients for antihormonal therapy. Appl Immunohistochem Mol Morphol 15:325–331
Viale G, Regan MM, Maiorano E, et al. (2008) Chemoendocrine compared with endocrine adjuvant therapies for node-negative breast cancer: predictive value of centrally reviewed expression of estrogen and progesterone receptors—International Breast Cancer Study Group. J Clin Oncol 26:1404–1410
Iwamoto T, Booser D, Valero V, et al. (2012) Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1 % to 10 % ER-positive by immunohistochemistry. J Clin Oncol 30:729–734
Acknowledgments
This study was funded by an unrestricted grant from Agendia. The authors would like to thank Annegien Broeks and Ingrid Hofland from the Core Facility Molecular Pathology and Biobank at the Netherlands Cancer Institute for their help with the IHC and SISH for central retesting.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
BN has received research funding from Agendia. KBD has received research funding from Alliance Research Centers. This study was funded by an unrestricted grant from Agendia. LD is an employee, and FdS, LS-S, and PR were employees of Agendia.
Informed consent
Patients provided written informed consent for their tumor samples to be evaluated in the study.
Rights and permissions
About this article
Cite this article
Wesseling, J., Tinterri, C., Sapino, A. et al. An international study comparing conventional versus mRNA level testing (TargetPrint) for ER, PR, and HER2 status of breast cancer. Virchows Arch 469, 297–304 (2016). https://doi.org/10.1007/s00428-016-1979-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-1979-9