Abstract
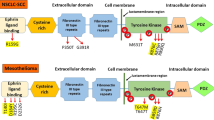
Malignant pleural mesothelioma (MPM) is the most common primary tumor of the pleura. Its incidence is increasing in Europe and the prognosis remains poor. We compared epithelioid MPM in short and long survivors, and identified signal transducer and activator of transcription 1 (STAT1) as probably being responsible for antiapoptotic signaling and chemoresistance. Six mesothelioma cell lines were evaluated by Western Blot. We also analyzed 16 epithelioid MPM tissue samples for the phosphorylation status of STAT1 and the expression of its negative regulator, the suppressor of cytokine signaling 1 (SOCS1). Formalin-fixed and paraffin-embedded tissue specimens were evaluated by protein-lysate microarray and immunohistochemistry. We found STAT1 to be highly expressed and STAT3 downregulated in MPM cell lines. The expression of STAT1 phosphorylated on tyrosine 701 (Y701) was increased by interferon-gamma (IFN-γ) treatment, whereas SOCS1 was not expressed. The expression of STAT1 phosphorylated on serine 727 (S727) was not detected in mesothelioma cell lines and was not stimulated by IFN-γ. STAT1 was phosphorylated on tyrosine 701 and serine 727 in MPM tissue samples. The expression of pSTAT1-Y701 was increased compared to pSTAT1-S727. SOCS1 was again not detectable. STAT1 is upregulated in MPM, and its action may be prolonged by a loss of the negative regulator SOCS1. STAT1 might, therefore, be a target for therapeutic intervention, with the intention to restore apoptotic mechanisms and sensitivity to chemotherapy. However, other regulatory mechanisms need to be investigated to clarify if lack of expression of SOCS1 is the only reason for sustained STAT1 expression in MPM.




Similar content being viewed by others
References
Vogelzang NJ, Porta C, Mutti L (2005) New agents in the management of advanced mesothelioma. Semin Oncol 32(3):336–350
Shukla A, Bosenberg MW, MacPherson MB, Butnor KJ, Heintz NH, Pass HI, Carbone M, Testa JR, Mossman BT (2009) Activated cAMP response element binding protein is overexpressed in human mesotheliomas and inhibits apoptosis. Am J Pathol 175(5):2197–2206. doi:10.2353/ajpath.2009.090400
Ramos-Nino ME, Testa JR, Altomare DA, Pass HI, Carbone M, Bocchetta M, Mossman BT (2006) Cellular and molecular parameters of mesothelioma. J Cell Biochem 98(4):723–734. doi:10.1002/jcb.20828
Nowak AK, Lake RA, Kindler HL, Robinson BW (2002) New approaches for mesothelioma: biologics, vaccines, gene therapy, and other novel agents. Semin Oncol 29(1):82–96
Boutin C, Nussbaum E, Monnet I, Bignon J, Vanderschueren R, Guerin JC, Menard O, Mignot P, Dabouis G, Douillard JY (1994) Intrapleural treatment with recombinant gamma-interferon in early stage malignant pleural mesothelioma. Cancer 74(9):2460–2467
Hand A, Pelin K, Mattson K, Linnainmaa K (1995) Interferon (IFN)-alpha and IFN-gamma in combination with methotrexate: in vitro sensitivity studies in four human mesothelioma cell lines. Anticancer Drugs 6(1):77–82
Hand AM, Husgafvel-Pursiainen K, Pelin K, Vallas M, Suitiala T, Ekman A, Mattson M, Mattson K, Linnainmaa K (1992) Interferon-alpha and -gamma in combination with chemotherapeutic drugs: in vitro sensitivity studies in four human mesothelioma cell lines. Anticancer Drugs 3(6):687–694
Hand AM, Husgafvel-Pursiainen K, Tammilehto L, Mattson K, Linnainmaa K (1991) Malignant mesothelioma: the antiproliferative effect of cytokine combinations on three human mesothelioma cell lines. Cancer Lett 58(3):205–210
Phan-Bich L, Buard A, Petit JF, Zeng L, Tenu JP, Chretien P, Monnet I, Boutin C, Bignon J, Lemaire G, Jaurand MC (1997) Differential responsiveness of human and rat mesothelioma cell lines to recombinant interferon-gamma. Am J Respir Cell Mol Biol 16(2):178–186. doi:10.1165/ajrcmb.16.2.9032125
Zeng L, Buard A, Monnet I, Boutin C, Fleury J, Saint-Etienne L, Brochard P, Bignon J, Jaurand MC (1993) In vitro effects of recombinant human interferon gamma on human mesothelioma cell lines. Int J Cancer 55(3):515–520
Buard A, Vivo C, Monnet I, Boutin C, Pilatte Y, Jaurand MC (1998) Human malignant mesothelioma cell growth: activation of janus kinase 2 and signal transducer and activator of transcription 1alpha for inhibition by interferon-gamma. Cancer Res 58(4):840–847
Rawlings JS, Rosler KM, Harrison DA (2004) The JAK/STAT signaling pathway. J Cell Sci 117(Pt 8):1281–1283. doi:10.1242/jcs.00963
Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, Russo A (2003) STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol 197(2):157–168. doi:10.1002/jcp.10364
Reich NC, Liu L (2006) Tracking STAT nuclear traffic. Nat Rev Immunol 6(8):602–612. doi:10.1038/nri1885
Sadzak I, Schiff M, Gattermeier I, Glinitzer R, Sauer I, Saalmuller A, Yang E, Schaljo B, Kovarik P (2008) Recruitment of Stat1 to chromatin is required for interferon-induced serine phosphorylation of Stat1 transactivation domain. Proc Natl Acad Sci USA 105(26):8944–8949. doi:10.1073/pnas.0801794105
Stephanou A, Latchman DS (2005) Opposing actions of STAT-1 and STAT-3. Growth Factors 23(3):177–182. doi:10.1080/08977190500178745
Kothmaier H, Quehenberger F, Halbwedl I, Morbini P, Demirag F, Zeren H, Comin CE, Murer B, Cagle PT, Attanoos R, Gibbs AR, Galateau-Salle F, Popper HH (2008) EGFR and PDGFR differentially promote growth in malignant epithelioid mesothelioma of short and long term survivors. Thorax 63(4):345–351. doi:10.1136/thx.2007.085241
Buettner R, Mora LB, Jove R (2002) Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res 8(4):945–954
Wormald S, Hilton DJ (2004) Inhibitors of cytokine signal transduction. J Biol Chem 279(2):821–824. doi:10.1074/jbc.R300030200
Espert L, Dusanter-Fourt I, Chelbi-Alix MK (2005) Negative regulation of the JAK/STAT: pathway implication in tumorigenesis. Bull Cancer 92(10):845–857
Klampfer L (2008) The role of signal transducers and activators of transcription in colon cancer. Front Biosci 13:2888–2899
Pass HI, Stevens EJ, Oie H, Tsokos MG, Abati AD, Fetsch PA, Mew DJ, Pogrebniak HW, Matthews WJ (1995) Characteristics of nine newly derived mesothelioma cell lines. Ann Thorac Surg 59(4):835–844
Reale FR, Griffin TW, Compton JM, Graham S, Townes PL, Bogden A (1987) Characterization of a human malignant mesothelioma cell line (H-MESO-1): a biphasic solid and ascitic tumor model. Cancer Res 47(12):3199–3205
Shukla A, Hillegass JM, MacPherson MB, Beuschel SL, Vacek PM, Pass HI, Carbone M, Testa JR, Mossman BT (2010) Blocking of ERK1 and ERK2 sensitizes human mesothelioma cells to doxorubicin. Mol Cancer 9:314. doi:10.1186/1476-4598-9-314
Ordonez NG (2007) What are the current best immunohistochemical markers for the diagnosis of epithelioid mesothelioma? A review and update. Hum Pathol 38(1):1–16
Becker KF, Schott C, Hipp S, Metzger V, Porschewski P, Beck R, Nahrig J, Becker I, Hofler H (2007) Quantitative protein analysis from formalin-fixed tissues: implications for translational clinical research and nanoscale molecular diagnosis. J Pathol 211(3):370–378
Kothmaier H, Rohrer D, Stacher E, Quehenberger F, Becker KF, Popper HH (2011) Comparison of formalin-free tissue fixatives: a proteomic study testing their application for routine pathology and research. Arch Pathol Lab Med 135(6):744–752
Shaffer J (1986) Modified sequentially rejective multiple test procudures. J Am Stat Assoc 81(395):826–831
Arzt L, Kothmaier H, Quehenberger F, Halbwedl I, Popper HH (2011) STAT signalling in malignant mesothelioma: is there a regulatory effect of microRNAs? Mag Eur Med Oncol 4(1):38–42
Ma J, Cao X (2006) Regulation of Stat3 nuclear import by importin alpha5 and importin alpha7 via two different functional sequence elements. Cell Signal 18(8):1117–1126
Wenta N, Strauss H, Meyer S, Vinkemeier U (2008) Tyrosine phosphorylation regulates the partitioning of STAT1 between different dimer conformations. Proc Natl Acad Sci USA 105(27):9238–9243
McBride KM, Banninger G, McDonald C, Reich NC (2002) Regulated nuclear import of the STAT1 transcription factor by direct binding of importin-alpha. Embo J 21(7):1754–1763
Kumar A, Commane M, Flickinger TW, Horvath CM, Stark GR (1997) Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science 278(5343):1630–1632
Mita AC, Mita MM, Nawrocki ST, Giles FJ (2008) Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res 14(16):5000–5005. doi:10.1158/1078-0432.ccr-08-0746
Kanwar RK, Cheung CH, Chang JY, Kanwar JR (2010) Recent advances in anti-survivin treatments for cancer. Curr Med Chem 17(15):1509–1515
Zimmerman M, Yang D, Hu X, Liu F, Singh N, Browning D, Ganapathy V, Chandler P, Choubey D, Abrams SI, Liu K (2010) IFN-gamma upregulates survivin and Ifi202 expression to induce survival and proliferation of tumor-specific T cells. PLoS One 5(11):e14076. doi:10.1371/journal.pone.0014076
Berishaj M, Gao SP, Ahmed S, Leslie K, Al-Ahmadie H, Gerald WL, Bornmann W, Bromberg JF (2007) Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Res 9(3):R32. doi:10.1186/bcr1680
Braun DA, Fribourg M, Sealfon SC (2013) Cytokine response is determined by duration of receptor and signal transducers and activators of transcription 3 (STAT3) activation. J Biol Chem 288(5):2986–2993. doi:10.1074/jbc.M112.386573
Russell MA, Cooper AC, Dhayal S, Morgan NG (2013) Differential effects of interleukin-13 and interleukin-6 on Jak/STAT signaling and cell viability in pancreatic beta-cells. Islets 5 (2)
Yang X, Liang L, Zhang XF, Jia HL, Qin Y, Zhu XC, Gao XM, Qiao P, Zheng Y, Sheng YY, Wei JW, Zhou HJ, Ren N, Ye QH, Dong QZ, Qin LX (2013) MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting IL-6-Stat3 pathway. Hepatology. doi:10.1002/hep.26305
Bromberg J (2002) Stat proteins and oncogenesis. J Clin Invest 109(9):1139–1142. doi:10.1172/jci15617
Aoki Y, Feldman GM, Tosato G (2003) Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 101(4):1535–1542. doi:10.1182/blood-2002-07-2130
Qing Y, Stark GR (2004) Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J Biol Chem 279(40):41679–41685. doi:10.1074/jbc.M406413200
Schindler C, Levy DE, Decker T (2007) JAK-STAT signaling: from interferons to cytokines. J Biol Chem 282(28):20059–20063. doi:10.1074/jbc.R700016200
Liao J, Fu Y, Shuai K (2000) Distinct roles of the NH2- and COOH-terminal domains of the protein inhibitor of activated signal transducer and activator of transcription (STAT) 1 (PIAS1) in cytokine-induced PIAS1-Stat1 interaction. Proc Natl Acad Sci USA 97(10):5267–5272
Ungureanu D, Vanhatupa S, Kotaja N, Yang J, Aittomaki S, Janne OA, Palvimo JJ, Silvennoinen O (2003) PIAS proteins promote SUMO-1 conjugation to STAT1. Blood 102(9):3311–3313. doi:10.1182/blood-2002-12-3816
Lee JH, Park SM, Kim OS, Lee CS, Woo JH, Park SJ, Joe EH, Jou I (2009) Differential SUMOylation of LXRalpha and LXRbeta mediates transrepression of STAT1 inflammatory signaling in IFN-gamma-stimulated brain astrocytes. Mol Cell 35(6):806–817. doi:10.1016/j.molcel.2009.07.021
Madonna S, Scarponi C, De Pita O, Albanesi C (2008) Suppressor of cytokine signaling 1 inhibits IFN-gamma inflammatory signaling in human keratinocytes by sustaining ERK1/2 activation. FASEB J 22(9):3287–3297
Lesinski GB, Zimmerer JM, Kreiner M, Trefry J, Bill MA, Young GS, Becknell B, Carson WE 3rd (2010) Modulation of SOCS protein expression influences the interferon responsiveness of human melanoma cells. BMC Cancer 10:142. doi:10.1186/1471-2407-10-142
Klampfer L (2006) Signal transducers and activators of transcription (STATs): novel targets of chemopreventive and chemotherapeutic drugs. Curr Cancer Drug Targets 6(2):107–121
Iwahori K, Serada S, Fujimoto M, Ripley B, Nomura S, Mizuguchi H, Shimada K, Takahashi T, Kawase I, Kishimoto T, Naka T (2013) SOCS-1 gene delivery cooperates with cisplatin plus pemetrexed to exhibit preclinical antitumor activity against malignant pleural mesothelioma. Int J Cancer 132(2):459–471. doi:10.1002/ijc.27611
Acknowledgments
This study is partly supported by the Styrian Cancer Aid Society and by an unrestricted grant from Pfizer Austria. We gratefully thank Hildegard Gleixner, Margit Gogg-Kamerer, Elisabeth Grygar, Mohammed Al-Effah, and Ralph König, Institute of Pathology, Medical University of Graz, for their excellent technical assistance.
Disclosure of interest
All authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arzt, L., Kothmaier, H., Halbwedl, I. et al. Signal transducer and activator of transcription 1 (STAT1) acts like an oncogene in malignant pleural mesothelioma. Virchows Arch 465, 79–88 (2014). https://doi.org/10.1007/s00428-014-1584-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1584-8