Abstract
During primitive streak formation in the chick embryo, cells undergo mesendoderm specification and convergent extension at the same time and in the same cells. Previous work has implicated cVG1 (GDF3) as a key factor for induction of primitive streak identity and positioning the primitive streak, whereas FGF signalling was implicated in regulating cell intercalation via regulation of components of the WNT-planar cell polarity (PCP) pathway. FGF has also been reported to be able to induce a primitive streak (but lacking the most axial derivatives such as notochord/prechordal mesendoderm). These signals emanate from different cell populations in the embryo, so how do they interact to ensure that the same cells undergo both cell intercalation and acquire primitive streak identity? Here we begin to address this question by examining in more detail the ability of the two classes of signals in regulating the two developmental events. Using misexpression of inducers and/or exposure to inhibitors and in situ hybridisation, we study how these two signals regulate expression of Brachyury (TBXT) and PRICKLE1 as markers for the primitive streak and the PCP, respectively. We find that both signals can induce both properties, but while FGF seems to be required for induction of the streak by cVG1, it is not necessary for induction of PRICKLE1. The results are consistent with cVG1 being a common regulator for both primitive streak identity and the initiation of convergent extension that leads to streak elongation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Formation of the primitive streak in early amniote embryos involves the acquisition of several distinct properties by the same cells at about the same time. These properties include expressing specific markers of primitive streak “identity” (such as Brachyury/TBXT, SNAI2, TBX6), epithelial-to-mesenchymal transition (EMT) leading to cell ingression and epithelial cell intercalation (convergent extension), leading to elongation of the primitive streak along the midline. In the chick embryo, primitive streak formation can be initiated from any position around the circumference of the disc-shaped embryonic epiblast. Therefore, some key questions remaining to be answered include the following: what mechanisms are responsible for the same cell population acquiring all of these properties, what signals regulate each of the properties and how are they integrated by the responding cells?
Previous studies in the chick embryo have shown that cVG1 (annotated GDF3 in the chicken genome, although synteny suggests that this gene should more appropriately be considered as the orthologue of GDF1), a member of transforming growth factor (TGF)-beta superfamily, is expressed in the posterior marginal zone and is an important factor for positioning the primitive streak (Bertocchini and Stern 2012; Shah et al. 1997; Skromne and Stern 2002). Grafting a cell pellet expressing cVG1 in non-posterior regions of pre-primitive-streak stage embryos can induce primitive streak formation with hierarchical gene expression (Shah et al. 1997; Skromne and Stern 2002), and inhibition of cVG1 using morpholino oligonucleotides impairs primitive streak formation (Bertocchini and Stern 2012). Fibroblast growth factor (FGF) signalling has been shown to cooperate with cVG1 to induce the primitive streak (Bertocchini et al. 2004).
Other studies showed that primitive streak elongation involves medio-lateral cell intercalation (convergent-extension) of future primitive streak cells (Voiculescu et al. 2007, 2014), and that this involves the WNT-planar cell polarity (PCP) pathway (Voiculescu et al. 2007) acting through myosin-II (Rozbicki et al. 2015). Three PCP-pathway components, Flamingo (CELSR), PRICKLE1 and VANGL2 were shown to play a role in medio-lateral intercalation in the epiblast based on their overlapping expression and knockdown experiments with morpholinos (Voiculescu et al. 2007). This was confirmed with experiments involving misexpression of Dishevelled lacking the DEP domain, which acts as a dominant-negative (Rothbacher et al. 2000): this generates a broad and thickened TBXT-expressing domain without a morphological “streak” (Voiculescu et al. 2007), revealing that specification of the primitive steak identity (mesendoderm specification) and the convergent extension of the epiblast cells can be separated.
FGF signalling from the hypoblast (a deep layer under the epiblast, which eventually will contribute to the yolk sac stalk (Stern and Downs 2012)) regulates cell movements by inducing the expression of the WNT/PCP pathway genes in cells that will later form the primitive steak (Foley et al. 2000; Voiculescu et al. 2007). However, pre-primitive-streak stage embryos from which the hypoblast layer has been removed can still generate the primitive streak (and even multiple primitive streaks), which elongate(s) appropriately (Bertocchini and Stern 2002). This result raises the question whether FGF signalling derived from the hypoblast is necessary for the induction of the WNT/PCP pathway, and if not, what other signals are involved.
Here we explore the relative roles of FGF and cVG1 in regulating the specification of primitive streak identity and PCP components involved in cell intercalation during primitive streak formation. Our results suggest that FGF/MAPK signalling is required for cVG1 to induce TBXT; FGF/MAPK can also induce the PCP component PRICKLE1 but it is not required for the induction of PRICKLE1 by cVG1.
Materials and methods
Embryo harvest and whole mount in situ hybridisation
Fertilised White Leghorn hens’ eggs were obtained from Henry Stewart Farm, UK, and incubated at 38 °C until appropriate stages of embryos were obtained. Embryos were harvested in Pannett-Compton saline (Pannett and Compton 1924), then cultured by the modified New culture method (New 1955; Stern and Ireland 1981). In experiments involving grafts of cell pellets or microbeads, the cultured embryos were fixed in 4% paraformaldehyde in phosphate buffered saline at 4 °C overnight, and then whole mount in situ hybridisation was conducted as previously described (Stern 1998; Streit and Stern 2001). The probes we used were FGF8 (Streit and Stern 1999), TBXT (Kispert et al. 1995) and PRICKLE1 (Voiculescu et al. 2007). Some embryos were embedded in paraffin wax and sectioned using a Zeiss Microm microtome (HM315) at 10 μm thickness as previously described (Streit and Stern 2001). Whole embryos and sections were observed under an Olympus SZH10 stereo microscope or an Olympus Vanox-T optical microscope, respectively, and then imaged with a QImaging Retiga 2000R camera.
Misexpression of proteins with transfected cell pellets
Misexpression of proteins was conducted as previously described (Lee et al. 2022). Briefly, HEK293T cells were cultured in 10% foetal bovine serum in Dulbecco’s Modified Eagle Medium and transfected using PEI as reported previously (Papanayotou et al. 2013). Expression plasmids were DMVg1 for cVG1 expression (Shah et al. 1997) and pMT23 (murine BMP4 (Dickinson et al. 1990)) for BMP4 expression. Then, transfected cells were cultured for 36–48 h at 37 °C in a hanging drop to make a cell pellet ranging in size from 500 to 1000 cells.
Delivery of proteins and inhibitor via microbeads
Fifty micrograms per millilitre of mouse FGF8b (Sigma-Aldrich, F6926) in 0.1% bovine serum albumin in phosphate-buffered saline was delivered using heparin-acrylic beads (Sigma-Aldrich, H5236), while 250 μM SU5402 (Calbiochem, 572,630), 1 mM U0126 (Calbiochem, 662,005) and 1 mM U0124 (Calbiochem, 662,006) in DMSO were loaded onto AG1X2-formate beads (Streit et al. 2000). In each case, the beads were incubated overnight at 4 °C in the protein or chemical at the concentrations stated above. Beads were washed in Pannett-Compton saline just before use. Embryos were fixed at 3 h, 6 h or 9 h after the graft or after overnight incubation.
Results and discussion
Expression of FGF8, TBXT and PRICKLE1 at the time of primitive streak formation
During its formation, cells of the primitive streak execute at least two cellular events at the same time, primitive-streak-cell specification and convergent extension. It has previously been shown that cVG1, WNT and FGF signals collaborate to specify primitive-streak cells (Bertocchini et al. 2004; Skromne and Stern 2001, 2002), while WNT/PCP pathway, as a downstream target of FGF, is responsible for driving convergent extension via epiblast cell intercalation (Voiculescu et al. 2007, 2014), executed via myosin-II (Rozbicki et al. 2015). It has also been shown that specification of primitive streak cells (and mesendoderm) is separable from elongation of the primitive streak by convergent extension (Voiculescu et al. 2007, 2014). However, it is less clear how these two cellular events are regulated so that they occur in the same cells. To find out what inducing factor is responsible for each downstream result, PCP pathway activation and mesoderm formation (specification of primitive-streak cells), we begun by re-investigating the spatial and temporal expression patterns of mRNA of some genes associated with these events. The expression of cVG1 has been extensively studied in our laboratory including several publications (see below); now we have also reanalysed the expression of FGF8, TBXT and PRICKLE1. cVG1 and FGF8 have been implicated as inducing factors for primitive streak formation (Bertocchini et al. 2004; Shah et al. 1997), while TBXT and PRICKLE1 are used as makers for mesendoderm/primitive-streak cells and for the WNT/PCP pathway, respectively (Voiculescu et al. 2007). Previous studies reporting their expression patterns have mainly relied on whole-mounts and there are sometimes large gaps in the stages that have been examined; we therefore chose to follow their expression in sections (to determine the precise layers of cells that express the genes) and in a more precise time-course.
Expression of cVG1 is initially (from EGK stage X) (Eyal-Giladi and Kochav 1976) restricted to the epiblast of the marginal zone, followed some hours later by expression in the newly forming mesendoderm of the primitive streak in the posterior area pellucida (Lee et al. 2020; Shah et al. 1997). FGF8 is initially (also from EGK stage X) expressed only in the lower layers, particularly in Koller’s sickle (which defines the boundary between marginal zone and area pellucida) and the hypoblast underlying the area pellucida (Streit et al. 2000), and later (EGK XIII) both in the area pellucida epiblast and hypoblast at EGK XIII (Fig. 1A and D). A previous RNAseq analysis of pre-primitive-streak stage embryos revealed that the only member of the FGF family with significant levels of expression at these stages is FGF8 (Lee et al. 2020). Although FGF12 and FGF13 are expressed at low level in the RNAseq study, these levels are too low even for detection by in situ hybridisation (Karabagli et al. 2002). These results suggest that the sickle and hypoblast are likely to be the only significant sources of FGF signals at these early stages.
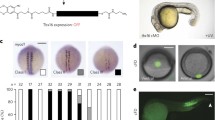
Expression patterns of FGF8, TBXT and PRICKLE1 in the pre-primitive-streak stage embryo, revealed by in situ hybridisation. FGF8 is detected exclusively in the hypoblast (hypo) at EGK stage XII (A), then in both the epiblast (epi) and the lower layers at EGK stage XIII (D). TBXT starts to be expressed in the posterior epiblast at EGK stage XII (B), then is also expressed in the forming mesendoderm at HH stage 2 (E). PRICKLE1 is expressed both in the epiblast and the lower layers of the primitive streak at EGK stage XIV (C and F). The upper (A–F) and lower (A’–F’ and A’’–F’’, 20 × and 40 × magnification, respectively) panels correspond, respectively, to a whole mount and a sagittal section at the level of the dashed line. Scale bar, 100 µm for all sections
Unexpectedly, we detect TBXT as early as stage EGK XI-XII (many hours prior to primitive streak formation) in the most posterior epiblast. As the primitive streak starts to appear (stage HH 2) (Hamburger and Hamilton 1951), TBXT is expressed in the forming mesendoderm of the streak including the overlying epiblast (Fig. 1B and E). PRICKLE1 starts to be expressed from stage EGK XII-XIII in the posterior area pellucida epiblast and Koller’s sickle and intensifies in primitive streak cells from stages EGK XIV–HH 2, both in the mesendoderm and in the overlying epiblast (Fig. 1C and F). These results are consistent with either cVG1 derived from the epiblast and/or FGF8 from the hypoblast being involved in the induction of TBXT (marking primitive streak identity) and/or PRICKLE1 (cells undergoing intercalation) expression in future primitive streak cells in the posterior area pellucida.
Timing of induction of PRICKLE1 and TBXT by cVG1 and FGF
Both cVG1 (Seleiro et al. 1996; Shah et al. 1997; Skromne and Stern 2002) and FGF8 (Bertocchini et al. 2004; Bertocchini and Stern 2012) induce primitive streak formation when misexpressed anteriorly or laterally. To investigate the dynamics of induction of PRICKLE1 and TBXT by cVG1, cVG1-expressing cell pellets were grafted to the anterior marginal zone of pre-primitive-streak stage (EGK X-XI) embryos and the expression of both genes was examined at different time points. Both genes start to be expressed by 9 h (but not 6 h) after the graft in the epiblast near the grafted cell pellet (Fig. 2). These results suggest that misexpression of cVG1 by graft of cell pellets can induce primitive-streak identity (TBXT) and the PCP pathway (PRICKLE1) in adjacent epiblast cells, with a similar time course.
cVG1 misexpression induces PRICKLE1 and TBXT expression. Two cVG1-expressing cell pellets were grafted to the anterior region of the embryo at EGK X-XI, and the expression of PRICKLE1 (A–D) and TBXT (E–H) was investigated by in situ hybridisation after 6, 9, 12 and 15 h incubation. Both genes begin to be expressed from 9 h after incubation in the epiblast above the grafted cell pellet. The upper (A–H) and lower (B’–D’ and F’–H’) panels show the whole embryo and a sagittal section at the level of the dotted lines. Arrows: location of the cVG1-expressing cell pellet. Scale bars, 100 µm for all sections. The number of embryos with ectopic expression and total number of embryos in (A–H) were 0/6, 5/6, 5/6, 4/5, 0/4, 5/5, 6/6 and 5/5
Next, we conducted equivalent experiments with FGF8. For this, a FGF8b-soaked bead was grafted onto the anterior marginal zone of pre-primitive-streak stage embryos (Fig. 3A); this has previously been reported to induce a primitive streak (Bertocchini et al. 2004) (Fig. 3B). PRICKLE1 was induced in adjacent cells 6 h after the graft (Fig. 3C and D), while weak expression of TBXT was first detectable after 3 h (Fig. 3E and F), increasing thereafter. These results show that both cVG1 and FGF8 can induce both TBXT (a marker of primitive streak identity) and PRICKLE1 (marker for the PCP) in adjacent tissues. In this experiment, FGF8 appears to induce expression of both genes more quickly than does cVG1.
FGF8 misexpression induces PRICKLE1 and TBXT. (A) Experimental design. An FGF8-soaked bead was grafted to the anterior region of the embryo at EGK X-XI. (B) After overnight culture, an ectopic primitive streak, expressing TBXT (arrow), was induced near the bead. (C–D) An FGF8-soaked bead induces PRICKLE1 by 6 h after grafting (arrow, D). (E–F) FGF8-beads also induce weak TBXT expression as soon as 3 h after grafting; this later intensifies (arrows, E and F). Number of embryos with ectopic expression and total number of embryos in (B–F): 4/6, 0/5, 3/5, 4/5 and 6/6
FGF signalling is not required for induction and maintenance of PRICKLE1, but is required for induction of TBXT
Previous studies have reported that the hypoblast and one of the signalling molecules it emits, FGF8, can influence the elongation of the primitive streak by regulating the WNT/PCP pathway, and thereby cell intercalation (Voiculescu et al. 2007, 2014). However, after removal of the hypoblast layer, embryos can still generate primitive streaks that undergo elongation (Bertocchini and Stern 2002). As the hypoblast (and Koller’s sickle) is the main source of FGF signalling at these early stages, this raises the question of whether FGF signalling is required for convergent extension during primitive streak formation. To address this, beads soaked in a FGF signalling inhibitor, SU5402 (Bertocchini et al. 2004; Mohammadi et al. 1997; Streit et al. 2000), were grafted either into the posterior area pellucida of the embryo (to assess the effect on normal development) (Fig. 4A), or into the anterior area pellucida together with a cVG1-expressing cell pellet (to test whether FGF signalling is required for cVG1 to induce a primitive streak and/or the PCP in an ectopic location) (Fig. 4F). SU5402 treatment inhibited TBXT expression in the posterior region; it also repressed the induction of TBXT by a cVG1 pellet (Fig. 4B, C, G and H), indicating that FGF signalling is required both for maintenance and for induction of TBXT. However, SU5402 treatment did not inhibit either the induction or the endogenous expression of PRICKLE1 in similar experiments (Fig. 4D, E, I and J), suggesting that FGF signalling is not required for PRICKLE1 expression. FGF signals are transduced through the mitogen‑activated protein kinase pathway via MEK1/2 (Huang et al. 1995; Kouhara et al. 1997). To confirm the results obtained with SU5402, we conducted comparable experiments with a selective inhibitor of MEK1/2, U0126 (using the inactive analogue U0124 as control) (Fig. 5) (Favata et al. 1998). U0126, but not the control, treatment yielded similar results to SU5402, suggesting that MAPK signalling is required for maintenance and induction of TBXT but not for PRICKLE1. Together, these results suggest that FGF/MAPK signalling is necessary for cVG1 to induce TBXT, but not for its induction of PRICKLE1.
Effect of SU5402, a FGF inhibitor, on the induction of TBXT and PRICKLE1. (A and F) Experimental design. SU5402-soaked or control (DMSO) beads were grafted either into the posterior area pellucida (four beads, A), or into the anterior area pellucida at EGK X-XI together with a cVG1-expressing cell pellet (two beads, F). Then, after 9-h incubation, expression of TBXT or PRICKLE1 was investigated by in situ hybridisation. (B–E) SU5402-soaked beads inhibit TBXT expression but not PRICKLE1 in the posterior area pellucida (prospective primitive streak cells). (G–J) SU5402-soaked beads inhibit induction of TBXT but not PRICKLE1 by a cVG1-pellet in the anterior area pellucida. Arrows, induced expression. Number of embryos with expression near the beads and total number of embryos: 0/4, 2/2, 4/4 and 3/3, respectively, for B–E, and 0/6, 4/4, 2/3 and 6/6, respectively, for G–J. Note that in some embryos, some of the beads become detached during fixation or in situ hybridisation
Effect of U0126, a MEK1/2 inhibitor, on the induction of TBXT and PRICKLE1. (A and F) Experimental design. U0126-soaked or control (U0124) beads were grafted into either the posterior area pellucida (four beads, A), or into the anterior area pellucida at EGK X-XI together with a cVG1-expressing cell pellet (two beads, F). Then, after 9-h incubation, expression of TBXT or PRICKLE1 was investigated by in situ hybridisation. (B–E) U0126-soaked beads, but not control (U0124) beads, inhibit TBXT expression but not PRICKLE1 posteriorly. (G–J) U0126-soaked beads inhibit induction of TBXT but not PRICKLE1 by cVG1 anteriorly. Arrows, induced expression. Number of embryos with expression near the beads and total number of embryos: 2/12, 8/9, 11/12 and 5/8, respectively, for B–E, and 0/4, 3/4, 4/5 and 5/5, respectively, for G–J. Note that as in Fig. 4, some of the beads have become detached during processing of the embryos
Finally, we tested the effects of misexpression of BMP4, an inhibitor of primitive streak formation that acts antagonistically to cVG1 (Bertocchini and Stern 2012; Streit et al. 1998). Grafts of BMP4-expressing cell pellets onto the posterior area pellucida (Fig. 6A) caused a gap in the domain of expression of PRICKLE1 (at 9 h) and generated a split primitive streak after overnight culture (Fig. 6B–E). This result is consistent with previous findings that cVG1 is required for primitive streak formation (Bertocchini and Stern 2012), and also suggest that cVG1 may be involved in regulating PRICKLE1 expression. One interesting possibility is that PRICKLE1 expression is normally repressed by BMP (which exists in the early embryo as a gradient, decreasing posteriorly), and that BMP activity can in turn be inhibited by either FGF (perhaps through phosphorylation of the linker region of Smad1; Demagny et al. 2014; Demagny and De Robertis 2016; Pera et al. 2003) or by Vg1/GDF (perhaps by competition for Smad4; Candia et al. 1997).
Inhibition of TBXT and PRICKLE1 by BMP4 misexpression. (A) Experimental design. A BMP4-expressing cell pellet is grafted to the posterior region of the embryo at EGK X-XI, and the expression of PRICKLE1 (after 9-h incubation) (B–C) or TBXT (after overnight incubation) (D–E) was investigated by in situ hybridisation. BMP4 misexpression splits the primitive streak forming region, sometimes generating a double primitive streak, one on either side of the grafted cell pellet (B, D). Dotted circles, location of the cell pellet. Number of embryos showing inhibitory effect and total number of embryos: 7/7, 0/5, 5/6 and 0/5, respectively, for B–E
Conclusion
Our results suggest that specific markers for two different cellular events (mesoderm specification and convergent extension) that characterise the cells destined to form the primitive streak are differentially regulated by the upstream signals, cVG1 and FGF8. As the cells in the primitive-streak forming region also undergo EMT and ingression while experiencing other signals (for example WNT, NODAL, BMP etc.), it will be of interest in future to study how those different signals interact to accomplish several cellular events.
Data availability
All data contributing to this paper will be made available by the authors upon request, without restrictions.
References
Bertocchini F, Stern CD (2002) The hypoblast of the chick embryo positions the primitive streak by antagonizing nodal signaling. Dev Cell 3:735–744
Bertocchini F, Stern CD (2012) Gata2 provides an early anterior bias and uncovers a global positioning system for polarity in the amniote embryo. Development 139:4232–4238
Bertocchini F, Skromne I, Wolpert L, Stern CD (2004) Determination of embryonic polarity in a regulative system: evidence for endogenous inhibitors acting sequentially during primitive streak formation in the chick embryo. Development 131:3381–3390
Candia AF, Watabe T, Hawley SH, Onichtchouk D, Zhang Y, Derynck R, Niehrs C, Cho KW (1997) Cellular interpretation of multiple TGF-beta signals: intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development 124:4467–4480
Demagny H, De Robertis EM (2016) Point mutations in the tumor suppressor Smad4/DPC4 enhance its phosphorylation by GSK3 and reversibly inactivate TGF-beta signaling. Mol Cell Oncol 3:e1025181
Demagny H, Araki T, De Robertis EM (2014) The tumor suppressor Smad4/DPC4 is regulated by phosphorylations that integrate FGF, Wnt, and TGF-beta signaling. Cell Rep 9:688–700
Dickinson ME, Kobrin MS, Silan CM, Kingsley DM, Justice MJ, Miller DA, Ceci JD, Lock LF, Lee A, Buchberg AM et al (1990) Chromosomal localization of seven members of the murine TGF-beta superfamily suggests close linkage to several morphogenetic mutant loci. Genomics 6:505–520
Eyal-Giladi H, Kochav S (1976) From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I General Morphology Dev Biol 49:321–337
Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM (1998) Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273:18623–18632
Foley AC, Skromne I, Stern CD (2000) Reconciling different models of forebrain induction and patterning: a dual role for the hypoblast. Development 127:3839–3854
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Huang J, Mohammadi M, Rodrigues GA, Schlessinger J (1995) Reduced activation of RAF-1 and MAP kinase by a fibroblast growth factor receptor mutant deficient in stimulation of phosphatidylinositol hydrolysis. J Biol Chem 270:5065–5072
Karabagli H, Karabagli P, Ladher RK, Schoenwolf GC (2002) Comparison of the expression patterns of several fibroblast growth factors during chick gastrulation and neurulation. Anat Embryol (berl) 205:365–370
Kispert A, Ortner H, Cooke J, Herrmann BG (1995) The chick Brachyury gene: developmental expression pattern and response to axial induction by localized activin. Dev Biol 168:406–415
Kouhara H, Hadari YR, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J (1997) A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89:693–702
Lee HC, Lu HC, Turmaine M, Oliveira NMM, Yang Y, De Almeida I, Stern CD (2020) Molecular anatomy of the pre-primitive-streak chick embryo. Open Biol 10:190299
Lee HC, Hastings C, Oliveira NMM, Perez-Carrasco R, Page KM, Wolpert L, Stern CD (2022) “Neighbourhood watch” model: embryonic epiblast cells assess positional information in relation to their neighbours. Development (2022) 149:dev200295. https://doi.org/10.1242/dev.200295
Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J (1997) Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276:955–960
New DAT (1955) A new technique for the cultivation of the chick embryo in vitro. J Embryol Exp Morph 3:326–331
Pannett CA, Compton A (1924) The cultivation of tissues in saline embryonic juice. Lancet 1:381–384
Papanayotou C, De Almeida I, Liao P, Oliveira NM, Lu SQ, Kougioumtzidou E, Zhu L, Shaw A, Sheng G, Streit A, Yu D, Wah Soong T, Stern CD (2013) Calfacilitin is a calcium channel modulator essential for initiation of neural plate development. Nat Commun 4:1837
Pera EM, Ikeda A, Eivers E, De Robertis EM (2003) Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev 17:3023–3028
Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE (2000) Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J 19:1010–1022
Rozbicki E, Chuai M, Karjalainen AI, Song F, Sang HM, Martin R, Knolker HJ, MacDonald MP, Weijer CJ (2015) Myosin-II-mediated cell shape changes and cell intercalation contribute to primitive streak formation. Nat Cell Biol 17:397–408
Seleiro EA, Connolly DJ, Cooke J (1996) Early developmental expression and experimental axis determination by the chicken Vg1 gene. Curr Biol 6:1476–1486
Shah SB, Skromne I, Hume CR, Kessler DS, Lee KJ, Stern CD, Dodd J (1997) Misexpression of chick Vg1 in the marginal zone induces primitive streak formation. Development 124:5127–5138
Skromne I, Stern CD (2001) Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development 128:2915–2927
Skromne I, Stern CD (2002) A hierarchy of gene expression accompanying induction of the primitive streak by Vg1 in the chick embryo. Mech Dev 114:115–118
Stern CD (1998) Detection of multiple gene products simultaneously by in situ hybridization and immunohistochemistry in whole mounts of avian embryos. Curr Top Dev Biol 36:223–243
Stern CD, Downs KM (2012) The hypoblast (visceral endoderm): an evo-devo perspective. Development 139:1059–1069
Stern CD, Ireland GW (1981) An integrated experimental study of endoderm formation in avian embryos. Anat Embryol 163:245–263
Streit A, Stern CD (1999) Mesoderm patterning and somite formation during node regression: differential effects of chordin and noggin. Mech Dev 85:85–96
Streit A, Stern CD (2001) Combined whole-mount in situ hybridization and immunohistochemistry in avian embryos. Methods 23:339–344
Streit A, Lee KJ, Woo I, Roberts C, Jessell TM, Stern CD (1998) Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development 125:507–519
Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD (2000) Initiation of neural induction by FGF signalling before gastrulation. Nature 406:74–78
Voiculescu O, Bertocchini F, Wolpert L, Keller RE, Stern CD (2007) The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature 449:1049–1052
Voiculescu O, Bodenstein L, Lau IJ, Stern CD (2014) Local cell interactions and self-amplifying individual cell ingression drive amniote gastrulation. Elife 3:e01817
Funding
This research was funded by a Wellcome Trust Investigator Award (107055/Z/15/Z) to CDS. HCL was supported by the same award and by a Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A6A3A03053468).
Author information
Authors and Affiliations
Contributions
HCL performed all experiments. NMMO provided technical assistance including transfection of cell pellets. HCL and CDS wrote the manuscript. CDS provided supervision and obtained funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, H.C., Oliveira, N.M.M. & Stern, C.D. Exploring the roles of FGF/MAPK and cVG1/GDF signalling on mesendoderm induction and convergent extension during chick primitive streak formation. Dev Genes Evol 232, 115–123 (2022). https://doi.org/10.1007/s00427-022-00696-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-022-00696-1