Abstract
Main conclusion
Expression levels of AtPP2-A3 and AtPP2-A8 are reduced in syncytia induced by Heterodera schachtii and decline of their expression levels decreases host susceptibility, whereas their overexpression promotes susceptibility to parasite.
Abstract
Plant-parasitic nematodes cause huge crop losses worldwide. Heterodera schachtii is a sedentary cyst-forming nematode that induces a feeding site called a syncytium via the delivery of secreted chemical substances (effectors) to host cells, which modulate host genes expression and phytohormone regulation patterns. Genes encoding the Nictaba-related lectin domain have been found among the plant genes with downregulated expression during the development of syncytia induced by H. schachtii in Arabidopsis thaliana roots. To investigate the role of two selected Nictaba-related genes in the plant response to beet cyst nematode parasitism, mutants and plants overexpressing AtPP2-A3 or AtPP2-A8 were infected, and promoter activity and protein localization were analyzed. In wild-type plants, AtPP2-A3 and AtPP2-A8 were expressed only in roots, especially in the cortex and rhizodermis. After nematode infection, their expression was switched off in regions surrounding a developing syncytium. Astonishingly, plants overexpressing AtPP2-A3 or AtPP2-A8 were more susceptible to nematode infection than wild-type plants, whereas mutants were less susceptible. Based on these results and changes in AtPP2-A3 and AtPP2-A8 expression patterns after treatments with different stress phytohormones, we postulate that the AtPP2-A3 and AtPP2-A8 genes play important roles in the defense response to beet cyst nematode infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant-parasitic nematodes are widespread root pests that cause high economic losses in crops (Jones et al. 2013). The most detrimental sedentary plant-parasitic nematodes belong to the groups of so-called cyst-forming and root-knot nematodes. Heterodera schachtii, also known as beet cyst nematode, is a representative of the former. Its infective juveniles (J2 stage) remain dormant inside egg shells stored in protective cysts. After hatching, they are attracted to host roots during migration through the soil. After entering rhizodermal cells, they migrate intracellularly toward the vascular cylinder, where they search for initial syncytial cells, which give rise to a specialized feeding site called a syncytium. The J2s pierce plant cell walls with a stylet and inject secretions produced in the nematode’s two subventral and one dorsal esophageal gland cells. These secretions are thought to be causative agents modifying plant cell metabolism and modulating changes in gene expression to facilitate development of the syncytium that sustains the nematode throughout its entire life cycle. Mature female cyst-forming nematodes are fertilized by vermiform mobile mature males; after oviposition, the females die, and their bodies become protective and highly resistant cysts (Sijmons et al. 1991). The syncytium develops through the formation of partial cell wall dissolutions followed by the fusion of protoplasts of adjacent hypertrophied cells of the vascular cylinder (Golinowski et al. 1996). In the roots of susceptible plants, fully developed and highly efficient syncytia are formed, allowing the development of mature female or male nematodes. In the roots of resistant plants, the syncytia are smaller and apparently less efficient. They usually degrade [due to induction of the hypersensitive response (HR)] or become surrounded by degraded neighboring cells, leading to degradation of the syncytium itself (Sobczak et al. 2005; Varypatakis et al. 2020). These syncytia are usually too short to support development of the females of the nematode, though mature males can develop.
Proteins that comprise the first line of defense of the plant immune system are extracellular surface pattern-recognition receptors (PRRs), containing among others leucine-rich repeat (LRR) domains, which recognize pathogens through activation by pathogen-associated molecular patterns (PAMPs). The proteins secreted by plant nematodes and products of cell wall degradation released during nematode migration may also be recognized as PAMPs. Activation of PRRs leads to a defense response called pattern-triggered immunity (PTI), which inhibits the proliferation of pathogens and disease spread. This phenomenon is referred to as basal resistance (Nürnberger and Lipka 2005). This kind of defense can be interrupted by effectors secreted by successful virulent pathogen isolates in plant cells (Dangl et al. 2013). Thus, the plant becomes susceptible, and the PAMP-induced defense is insufficient to suppress infection and colonization by pathogens. To counteract the action of pathogen effectors, plants have developed resistance (R) genes encoding members of the polymorphic superfamily of intracellular NBS-LRR receptors. NBS-LRR proteins possess characteristic domains such as NBS-LRR (nucleotide-binding site-leucine-rich repeats), NB-ARC (nucleotide-binding adaptor shared by Apaf-1, R proteins, and CED-4) and highly variable domains at their N-terminus, such as TIR (Toll-like/interleukin receptor), CC (coiled coil), RPW8 (resistance to powdery mildew 8), AIG1-type G (avrRpt2-induced), and other domains (Reuber and Ausubel 1996; Arya and Acharya 2018). Particular NBS-LRR proteins are activated by specific pathogen effectors via direct interaction or indirect activation when effectors modify host cellular targets, which then activate NBS-LRR proteins (Dangl et al. 2013). Activation of NBS-LRRs induces a defense response called effector-triggered immunity (ETI), which limits pathogen proliferation (Dangl et al. 2013). In the case of a few examined nematode resistance genes, it seems that NBS-LRRs do not act alone but employ a helper protein, another NBS-LRR. An example of helper proteins found in Solanaceae plants is HR-associated cell death (NRC) proteins. This type of cooperation has been confirmed for tomato Mi-1.2, an R protein, which depends on the presence of NRC4, and for potato Gpa2, an R protein depending upon NRC2 and NRC3 (reviewed by Goverse and Mitchum 2022). To date, only a few nematode R genes have been identified in plants and cloned. These genes, mostly originating from wild relatives of crops, have been introgressed into crop plant genomes by crossbreeding, providing useable cultivars. However, under natural field conditions, the resistance can often be overcome by selected virulent/resistance-breaking nematode populations that develop new (sets of) effectors. In A. thaliana (ecotype Columbia), the genomic sequences of 149 genes encoding NBS-LRRs have been identified (Meyers et al. 2003). According to Szakasits et al. (2009), the majority of A. thaliana NBS-LRR or disease-related genes are downregulated in syncytia at 5 and 15 days post-infection (dpi) with H. schachtii. This suggests that H. schachtii suppresses the defense responses of A. thaliana.
One of the most intriguing research topics in plant immunity is proteins that contain domains typical for plant immunity proteins but are possibly not R proteins themselves. N-terminal motifs characteristic of NBS-LRR proteins can also be found, among others, in members of the PP2-like protein family. By exploring the results of the syncytial transcriptome analysis performed by Szakasits et al. (2009), we found that PP2-like genes are strongly up- or downregulated during H. schachtii infestation of A. thaliana roots (described herein). The PP2-like protein family belongs to the larger lectin family and shares homology with Nicotiana tabacum L. agglutinin (abbreviated as Nictaba) (Eggermont et al. 2017). In total, 217 putative genes with lectin domains have been identified in the A. thaliana genome. They belong to 9 of 12 different lectin families (Eggermont et al. 2017). Analysis of domains in lectin proteins revealed that they contain at least one lectin domain, which can bind reversibly to specific carbohydrate motifs, free carbohydrates, and glycans from glycoproteins and glycolipids. The lectin domain can be linked to other domains, which have also been identified in proteins playing a role in stress signaling and plant defense (Eggermont et al. 2017). In the Arabidopsis genome, 30 orthologs of Nictaba (PP2-like) proteins have been identified, though none contain a signal peptide or transmembrane region (Eggermont et al. 2017). The majority of AtPP2-like/Nictaba proteins possess an F-box domain, four (including AtPP2-A8) orthologs contain an N-terminal Toll/interleukin-1 receptor (TIR) domain, and only one putative Nictaba lectin (AtPP2-A3) possesses an AIG1-type G domain (Eggermont et al. 2017).
Based on structural features and domains of the proteins, as well as on changes in gene expression in syncytia induced by the beet cyst nematode in Arabidopsis roots (Szakasits et al. 2009), we selected two Arabidopsis PP2-like genes (AtPP2-A8 and AtPP2-A3) for detailed analyses. The AtPP2-A8 protein possesses a Toll/Il-1R (TIR) domain with significant similarity to the TIR domain of the N resistance protein from Nicotiana glutinosa, conferring resistance to tobacco mosaic virus (TMV) via pathogen recognition and activation of defense responses (Whitham et al. 1994; Burch-Smith and Dinesh-Kumar 2007). In plants, the TIR domain is involved in the initial interaction with specific ligands that activate intracellular signaling cascades in response to pathogens (Van Der Biezen and Jones 1998). The AtPP2-A3 protein shows high similarity to the defense protein AIG1, which possesses a G domain (AIG1-type G domain) also found in GTPases (Reuber and Ausubel 1996).
The detailed role of PP2-like proteins is not sufficiently understood, similar to their hypothetical involvement in the plant response to soil-borne root pathogenic nematode attack. Therefore, the aim of this work was to provide a detailed functional analysis of two PP2-like genes (AtPP2-A3 and AtPP2-A8) in the Arabidopsis response to cyst nematode infestation.
Materials and methods
Plant materials and culture conditions
The wild-type (WT) Arabidopsis thaliana plants ecotype Columbia (Col-0) were used as control in all experiments. AtPP2-A3 overexpressing transgenic lines (PP2-A3oe7/1, PP2-A3oe9/5 and PP2-A3oe10/7), and AtPP2-A8 overexpressing lines (PP2-A8oe2/5, PP2-A8oe5/3 and PP2-A8oe8/2), and mutants (pp2-a3-1, pp2-a3-2 and pp2-a8-1) used in this study were also in Col-0 background. Seeds of the T-DNA pp2-a3 and pp2-a8 insertional mutants [SALK_045411 and SALK_027300 for AtPP2-A3 (AT2G26820) and SALK_074249 for AtPP2-A8 (AT5G45070)] were obtained from the Nottingham Arabidopsis Stock Centre (UK). Seeds were sterilized and plants were cultured in vitro on 0.2KNOP or 0.5MS media under conditions described by Wiśniewska et al. (2022).
Gene construct preparation and A. thaliana transformation
Complementary DNA isolated from segments of uninfected roots of 14-day-old A. thaliana plants was used to amplify 1584-bp-long coding sequence of AtPP2-A3 with UTR3’ fragment (NM_001336092.1 splicing form) with the forward primer CACC/ ATGTCAGAGCCAATCAAAAAC and reverse primer CGTTACCACGAAATTTAGA. Forward primer CACC/ ATGGCTGCTTCTTCTTCTGTGAGA and reverse primer TTACTGCGCTGGACGAATTGCAAAG were used to amplify 1065-bp-long coding sequence of AtPP2-A8. The polymerase chain reaction (PCR) products were cloned into the pENTR™/D-TOPO® vector (Thermo Scientific, Waltham, MA, USA) and verified by Sanger sequencing. The coding sequences were subcloned into a pK7WG2D vector (https://gatewayvectors.vib.be, accession date 10/28/2022) using Gateway® LR Clonase® II Enzyme mix (Thermo Scientific). The desired constructs were transferred into Agrobacterium tumefaciens EHA105 strain by electroporation (MicroPulser; Bio-Rad, Hercules, CA, USA).
Genomic DNA was isolated from frozen A. thaliana Col-0 leaves using the Genomic Mini AX Plant kit (A&A Biotechnology, Gdańsk, Poland). Genomic sequences of AtPP2-A3 and AtPP2-A8 genes promoters were amplified using primers pairs: CACC/ TTCCTATCCTTTTCTTTTCTGACTTC and ATCGAAGAGGAGAAAAGAAAGAAC (798 bp), and CACC/ AGGGTGAACGCAAAACCTAC and AAGAGAGCTTTTTCTTTTGAGGT (1217 bp), respectively. The PCR products were cloned into pENTR™/D-TOPO® vector (Thermo Scientific) and sequenced using Sanger method. The promoter fragments were subcloned into pKGWFS7 vector containing the kanamycin resistance gene (nptII) and GFP and GUS reporter genes (https://gatewayvectors.vib.be, accession date 10/28/2022) using Gateway® LR Clonase® II Enzyme mix (Thermo Scientific).
Gene constructs containing PP2-A3 and PP2-A8 promoters and coding sequences fused with the gene encoding GFP (pPP2-A3::PP2-A3-GFP and pPP2-A8::PP2-A8-GFP) were obtained using the In-Fusion HD Clontech kit (Takara, Kusatsu, Japan) according to the manufacturer's instructions using the appropriate primers (Table 1). They were cloned into the pK7FWG,0 vector (https://gatewayvectors.vib.be, accession date 10/28/2022) linearized previously with the EcoRV restrictase.
Transgenic overexpression lines (PP2-A3oe and PP2-A8oe) containing coding sequences fused to constitutive 35S promoter, transgenic reporter lines with promoters of AtPP2-A3 or AtPP2-A8 fused with the coding sequence of GUS reporter gene (pPP2-A3::GUS and pPP2-A8::GUS), and protein–GFP fusion lines with native promoters of PP2s genes (pPP2-A3::PP2-A3-GFP and pPP2-A8::PP2-A8-GFP) were obtained by the floral dip transformation method (Clough and Bent 1998). Genotype homozygosity was confirmed using the ratio of kanamycin-resistant to non-resistant T2 plants germinating on kanamycin-containing medium.
Mutants’ genotyping
The homozygosity of the T-DNA insertion lines was confirmed in the relevant DNA sequence sites using PCR and the mutant site-specific primers (Table 2) with SALK_LBb1.3:ATTTTGCCGATTTCGGAAC under the standard PCR conditions recommended by the SALK collection.
RNA extraction and reverse transcription PCR (RT-PCR)
Samples for RNA extraction were collected from segments of uninfected roots and root segments containing syncytia at 5 and 15 days post-infection (dpi) induced in WT, mutants, overexpression lines, and roots of uninfected WT plants treated with JA, (–)-methyl jasmonate (MeJA), SA, and abscisic acid (ABA) (Wiśniewska et al. 2021). Total RNA was isolated and cDNA was synthesized for expression analyses as described by Wiśniewska et al. (2021). Quantitative real-time PCR (qRT-PCR) was performed using SYBR Green Master Mix (Roche, Basel, Switzerland) and 1 μL cDNA as the template for analysis of gene expression in the uninfected and infected roots. Reactions were performed on a LightCycler 96 (Roche). Primers sets: F: GATTGCACAAGCGGAAGCAA and R: AGGTTCATTTTCACCGCTGC, and F: GCAGACATCGAAATTGCAACGA and R: TTCAGCGTCAGGGTCACTTC were used for expression analyses of AtPP2-A3 and AtPP2-A8 genes, respectively. Genes encoding dimethylallyl, adenosine tRNA methylthiotransferase (AT4G33380) (Czechowski et al. 2005) (primer F: TTGAAAATTGGAGTACCGTACCAA and R: TCCCTCGTATACATCTGGCCA), Actin2 (AT3g8780) (primer F: CTTGCACCAAGCAGCATGAA and R: CCCCAGCTTTTTAAGCCTTTGATC), Actin8 (AT1G49240) (primer F: ATGAAGATTAAGGTCGTGGCA and R: TCCGAGTTTGAAGAGGCTAC), and GAPDH (AT1G13440) (primer F: TTGGTGACAACAGGTCAAGCA and R: AAACTTGTCGCTCAATGCAATC) were used as references to calculate changes in genes expression. Relative gene expression was calculated according to method of Graeber et al. (2011).
Nematode infection assay
Infective second-stage juveniles (J2s) of beet cyst nematode (Heterodera schachtii) were obtained and plants were inoculated as described previously (Sijmons et al. 1991; Wiśniewska et al. 2021).
GUS activity assay
Histochemical detection of GUS activity was performed as described by Wiśniewska et al. (2013). GUS activity was examined in seedlings, leaves, flowers, siliques, non-infected roots, as well as in the roots containing 15 dpi syncytia induced by J2s of H. schachtii in roots of different genotypes of A. thaliana.
Confocal laser scanning microscopy
Microscopic examinations of transgenic A. thaliana lines containing GFP-PP2 proteins’ fusion under control of native promoters (pPP2-A3::PP2-A3-GFP and pPP2-A8::PP2-A8-GFP) were performed with a Leica TCS SP5II inverted confocal laser scanning microscope (CLSM) (Leica Microsystems, Wetzlar, Germany) on uninfected and nematode infected roots containing 5 dpi syncytia induced by H. schachtii. GFP fluorescence emission was monitored in 499–550-nm beam path after excitation with 488-nm line of an argon ion laser.
Statistical analyses
The significance of differences between means was tested using Fisher’s multiple range test and one-way analysis of variance (ANOVA). The least significant difference (LSD) was calculated at P < 0.05. Assumptions of ANOVA, i.e., homogeneity of variances and normality, were checked using statistical tests, Levene’s test, and Shapiro–Wilk test. The p values of these tests were greater than 0.05, which means that the assumptions were fulfilled. All the analyses were performed using Statgraphics software. Gene expression experiments were performed in triplicate. The nematode infection assay was performed in at least five biological replicates per genotype (n > 30).
Results
AtPP2-A3 and AtPP2-A8 gene expression in infected Arabidopsis roots
A. thaliana possesses 30 genes encoding orthologs of Nictaba lectins (PP2-like proteins) (Dinant et al. 2003; Eggermont et al. 2017). The analyses of gene expression in syncytia induced by the beet cyst nematode described by Szakasits et al. (2009) showed that two PP2-like genes (AtPP2-B1 and AtPP2-B11) were significantly upregulated and four (AtPP2-A3, AtPP2-A8, AtPP2-A6, and AtPP2-A14) significantly downregulated. Of the four downregulated genes, we selected two (AtPP2-A3 and AtPP2-A8) with the most decreased transcript accumulation levels (log2 value − 7.2 and − 4.9, respectively) for detailed examination. We used RT-qPCR to confirm the results of Szakasits et al. (2009). The expression level of AtPP2-A3 in roots containing 5 and 15 dpi syncytia was decreased to 60% (statistically significant) and 69%, respectively, of the expression level in uninfected roots of WT plants (Fig. 1a). The expression level of AtPP2-A8 was significantly reduced to 36% in infected roots at 5 dpi and 58% in roots with syncytia at 15 dpi in comparison to uninfected roots of WT plants (Fig. 1b). The results of gene expression analysis confirmed downregulation of AtPP2-A3 and AtPP2-A8 genes after nematode infection of A. thaliana roots.
Relative expression levels of AtPP2-A3 and AtPP2-A8 genes in syncytia induced by H. schachtii in wild-type A. thaliana. a Relative transcript levels of AtPP2-A3 in roots containing syncytia at 5 and 15 dpi. b Relative transcript levels of AtPP2-A8 in roots containing syncytia at 5 and 15 dpi. Bars show mean values ± SD. Asterisks indicate means ± SD (n = 3), which are significantly different at *P < 0.05 according to one-way ANOVA and post hoc Fisher’s least significant difference (LSD)
Expression of AtPP2-A3 and AtPP2-A8 genes in roots treated with phytohormones
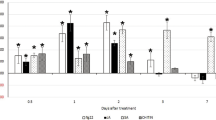
Expression levels of AtPP2-A3 and AtPP2-A8 genes were examined in the uninfected roots of WT plants after 24 h of exposure to jasmonic acid (JA), (-)-methyl jasmonate (MeJA), salicylic acid (SA), or abscisic acid (ABA). The level of AtPP2-A3 transcript accumulation decreased significantly after treatment with JA, MeJA and ABA in comparison to the water-treated control (Fig. 2a). SA did not influence AtPP2-A3 expression levels. The level of AtPP2-A8 transcript accumulation did not change after JA and MeJA treatment but increased significantly after application of SA and ABA (Fig. 2b). Based on the results, we confirmed that JA, MeJA, and ABA downregulated AtPP2-A3, but that SA and ABA upregulated AtPP2-A8. This difference in expression level changes of both genes after treatment with specific hormones may indicate that their expression is regulated by different mechanisms and regulatory pathways.
Relative expression levels of AtPP2-A3 (a) and AtPP2-A8 (b) genes in uninfected roots of wild-type A. thaliana treated with jasmonic acid (JA), (–)-methyl jasmonate (MeJA), salicylic acid (SA), or abscisic acid (ABA) (24 h treatment with 100 µM concentration each). Bars show mean values ± SD (n = 3). Different letters on bars indicate statistically significant differences according to one-way ANOVA (P < 0.05) and post hoc Fisher’s least significant difference (LSD)
Development of H. schachtii on roots of mutant and overexpressing Arabidopsis plants
The potential role of AtPP2-A3 and AtPP2-A8 genes in infection of A. thaliana roots and development of syncytia induced by beet cyst nematode (H. schachtii) was explored using T-DNA mutants (pp2-a3-1, pp2-a3-2, and pp2-a8-1), overexpressing lines (PP2-A3oe7/1, PP2-A3oe9/5, PP2-A3oe10/7, PP2-A8oe2/5, PP2-A8oe5/3, and PP2-A8oe8/2) and Col-0 wild-type plants. Downregulation of AtPP2s expression in mutant roots and upregulation in overexpressing lines were confirmed by RT-PCR (Fig. S1). In the pp2-a3-2 mutant, significantly lower average numbers of sedentary J2s at 5 dpi (by approximately 45%) (Fig. 3a) and significantly lower average numbers of developed females at 15 dpi for pp2-a3-1, pp2-a3-2, and pp2-a8-1 mutants (by approximately 34, 40, and 32%, respectively) (Fig. 3b) per root system were found. Average numbers of males developed on these genotypes at 15 dpi did not differ in comparison to control plants (Fig. 3c). In contrast, the average numbers of females (Fig. 3e) developing on the roots of AtPP2-A3- and AtPP2-A8-overexpressing lines at 15 dpi were significantly elevated (by approximately 40% for both overexpressed genes) compared to WT plants. However, average numbers of sedentary J2s at 5 dpi (Fig. 3d) and males at 15 dpi (Fig. 3f) showed no significant differences between WT and overexpressing lines. These results suggest that disruption of AtPP2-A3 and AtPP2-A8 expression decreases the susceptibility of A. thaliana to beet cyst nematode, but that their overexpression elevates susceptibility when expressed as the number of fully developed females. This clearly indicates that proper modulation of AtPP2-A3 and AtPP2-A8 gene expression plays a significant role in the response of A. thaliana roots to parasitism by H. schachtii.
H. schachtii development test comparing the susceptibility of wild-type, mutant, and AtPP2-overexpressing A. thaliana lines at 5 and 15 dpi. a Average numbers of sedentary second-stage juveniles developed on mutant roots at 5 dpi. b Average numbers of females developed on mutant roots at 15 dpi. c Average numbers of males developed on mutant roots at 15 dpi. d Average numbers of sedentary second-stage juveniles developed on overexpressing roots at 5 dpi. e Average numbers of females developed on overexpressing roots at 15 dpi. f Average numbers of males developed on overexpressing roots at 15 dpi. Bars show means ± SD (n > 30). Different letters on bars indicate statistically significant differences according to one-way ANOVA (P < 0.05) and post hoc Fisher’s least significant difference (LSD)
Expression pattern of AtPP2-A3 and AtPP2-A8 genes in A. thaliana
Two types of gene constructs for Arabidopsis transformation were prepared to determine activities of AtPP2-A3 and AtPP2-A8 gene promoters and patterns of their protein localization in uninfected and H. schachtii-infected plants. The first type of gene construct contained native promoters linked to a gene encoding β-glucuronidase (GUS) (Fig. 4a–l). The second type of construct contained the coding sequence of AtPP2-A3 or AtPP2-A8 linked in a single open-reading frame with the GFP gene under the control of native AtPP2-A3 or AtPP2-A8 promoter fragments (Fig. 4m–t).
Microscopic analyses of AtPP2-A3 and AtPP2-A8 expression in Arabidopsis plants. Stereo-light microscope (a–l) and confocal laser scanning (m–t) images of transgenic plants expressing the GUS reporter gene (bluish coloration) (a–l) or GFP reporter gene (greenish coloration) (m-t) under control of the AtPP2-A3 (a, b, e, f, i, j, m, n, q and r) or AtPP2-A8 (c, d, g, h, k, l, o, p, s and t) promoter. a and c Expression in 12-day-old seedlings. b and d Expression in inflorescence and siliques. e, g and m–p Expression in uninfected roots. f, i–l and q–t Expression in roots and around syncytia induced by beet cyst nematode. (f and h–l 15 dpi; r-t 5 dpi). F flower, N nematode, Nu nucleus, RT root tip, S syncytium, Si silique. Scale bars: 1 mm (a–d); 250 µm (e–h, o); 100 µm (i–n, p, q, s); 20 µm (r, t)
Overall, the spatial patterns of AtPP2-A3 and AtPP2-A8 gene expression were similar in the organs and tissues of uninfected A. thaliana seedlings (Fig. 4a–e and g). In leaves, inflorescence stems, flower buds, flowers, and siliques, the promoters of the AtPP2-A3 or AtPP2-A8 genes were inactive (Fig. 4a–d). In uninfected roots, GUS activity was found to be very high, as indicated by the intensity of GUS staining in outer tissues of older fragments of roots (rhizodermis and cortex), except for the apical parts of roots (root tips) and primordia of lateral roots (Fig. 4a, c, e and g). More intense coloration of roots expressing GUS under control of the AtPP2-A8 gene promoter indicated its higher expression level when compared to the AtPP2-A3 gene promoter (Fig. 4e, f, i and j versus g, h, k and l).
After root infection with the beet cyst nematode juveniles, the promoter activity of AtPP2-A3 or AtPP2-A8 genes indicated by GUS-produced coloration was absent in nematode feeding sites containing nematode-induced syncytia (Fig. 4f and h–l). The results of the GUS activity assay confirmed the previously obtained results of qRT-PCR analyses.
The use of gene constructs in which sequences of AtPP2-A3 or AtPP2-A8 genes were fused with the gene encoding the GFP reporter protein under the control of native gene promoters allowed us to obtain more precise information about localization of the proteins (Fig. 4m–t). In uninfected roots, GFP fused with AtPP2-A3 protein was observed mainly in the cytoplasm of root rhizodermis cells (Fig. 4m, n), whereas GFP fused with AtPP2-A8 protein was observed in the cytoplasm and nuclei of rhizodermal and cortical cells (Fig. 4o, p). In roots infected with beet cyst nematode, AtPP2-A3 or AtPP2-A8 protein synthesis was apparently decreased in cells in nematode-induced syncytia and surrounding cells, which, without nematode infection, accumulated the GFP fusion protein (Fig. 4q–t).
Expression pattern analysis of AtPP2-A3 or AtPP2-A8 genes revealed expression restricted to outer tissues of roots. Root infestation by H. schachtii led to a decrease in expression levels of both genes at infection sites. We also detected AtPP2-A8 localization in nuclei, which suggests that this gene might play a role in the regulation of transcription.
Discussion
A protein family consisting of 30 amino acid sequences was identified in the A. thaliana genome based on homology to the N. tabacum L. agglutinin (Nictaba) family, also known as lectins (Eggermont et al. 2017). The same 30 Arabidopsis proteins were previously identified as phloem proteins (P-proteins) based on their homology to CbmPP2 (PP2) from winter squash (Cucurbita maxima) and called PP2-like (Phloem Protein 2-like) (Dinant et al. 2003). Unfortunately, no evidence for their synthesis or occurrence in phloem sieve elements or companion cells has been provided thus far. Assigning names to new genes based only on their homology to functionally analyzed genes may result in misinterpretation of the actual role and localization of their putative proteins. The term P-proteins covers all types of specific proteins present in mature and differentiating phloem sieve elements of angiosperm plants (Cronshaw and Esau 1967). Cronshaw and Esau (1967) studied the ultrastructure of N. tabacum L. sieve element protoplasts and showed that the P-protein tubules reorganize into shorter striated tubules, which are characteristic of mature sieve elements. Based on this, the tubular P-protein component was designated P1-protein (known currently as Phloem Protein 1, PP1), and the fibrillar component was designated P2-protein (Phloem Protein 2, PP2). Unfortunately, the role and expression patterns of most Arabidopsis Nictaba proteins are unclear, and our analyses of AtPP2-A3 and AtPP2-A8 protein localization and their native gene promoter activity do not confirm that they are phloem proteins.
Expression pattern of AtPP2-A3 and AtPP2-A8 genes in Arabidopsis roots
The pattern of activity of both tested AtPP2-A3 and AtPP2-A8 promoters in Arabidopsis tissues was similar, being mainly limited to root tissues, with no signals of activity found in the other organs, such as leaves, flower buds, flowers, or siliques. Detailed analyses using transgenic plants expressing two types of gene constructs showed that promoter activity and synthesis of PP2-A3 and PP2-A8 proteins occurs mainly in the root rhizodermis and cortex but not in phloem cells, as might be supposed by the original names of both genes. These results were astonishing, because the gene promoter activity of two other previously described Arabidopsis PP2-like genes (AtPP2-A1 and AtPP2-A2) was detected in vascular tissue (Dinant et al. 2003). Activity of the circa 1 kb-long AtPP2-A1 gene promoter was detected in the vascular tissue of Arabidopsis and in the phloem of the bicollateral vascular bundles of tobacco (N. tabacum) stems. A similar pattern of expression, but at lower levels, was observed for the 1 kb-long AtPP2-A2 gene promoter in transgenic Arabidopsis. These results were confirmed by in situ mRNA hybridization experiments. AtPP2-A1 and AtPP2-A2 mRNAs were found in companion cell-sieve element complexes, mostly in companion cells (Dinant et al. 2003). However, AtPP2-B10 gene promoter activity was shown in young leaf trichomes, petioles, major leaf veins, and some mesophyll regions in older leaves (Stefanowicz et al. 2016).
To determine the cellular localization of the products of the AtPP2-A3 and AtPP2-A8 genes, C-terminal GFP fusion constructs were used. The AtPP2-A3-GFP and AtPP2-A8-GFP fusion proteins localized to the cytoplasm and nuclei of Arabidopsis rhizodermis and root cortex cells. Other Nictaba proteins, i.e., AN4 (AtPP2-A9), AN5 (AtPP2-A1) and AtPP2-A5, have also been found in the cytoplasm and nucleus of Arabidopsis, whereas AN3 (AtPP2-A2) is only found in the cytoplasm (Eggermont et al. 2018; Santamaría et al. 2019).
Differential expression of AtPP2-A3 and AtPP2-A8 in response to hormone treatments
Plants use phytohormones to respond to different environmental stresses. The signaling pathways controlled by SA and JA are mainly involved in the response to biotic stress, whereas abiotic stress responses are usually controlled by ABA-related pathways. The role of ABA in response to biotic stress is discussed. To establish feeding sites, plant-parasitic nematodes change plant cell metabolism, among others, by modulating hormonal cross-talk and interactions with hormone pathways (Gheysen and Mitchum 2019).
AtPP2-A3 and AtPP2-A8 genes were downregulated after nematode infection and had similar tissue expression patterns, but their expression levels differed after hormone treatments. The expression level of the AtPP2-A3 gene was downregulated by JA, MeJA and ABA treatment, whereas expression of AtPP2-A8 was upregulated by SA and ABA.
Expression of other Nictaba genes, AN3 (AtPP2-A2), AN4 (AtPP2-A9), and AN5 (AtPP2-A1), is also differentially regulated (Eggermont et al. 2018). Transcript accumulation of AN3 was significantly upregulated after treatment with MeJA, ABA, and SA. The expression level of AN4(AtPP2-A9) was downregulated after MeJA and ABA treatment, and SA treatment practically did not change its expression level. The expression level of AN5(AtPP2-A1) was slightly upregulated by MeJA treatment and stronger after ABA treatment, and SA treatment practically did not affect its expression (Eggermont et al. 2018).
Sedentary plant-parasitic nematodes need auxin and cytokinin to induce development and to maintain their feeding sites (Gheysen and Mitchum 2019). Stress hormones, such as SA, activate basal defense mechanisms against plant-parasitic nematodes. Although the role of JA in the response to parasitic nematodes is still being discussed, available data indicate that it strongly depends on nematode species, host plant species, and even particular plant genotypes (resistant or susceptible) (Gheysen and Mitchum 2019). Downregulation of AtPP3-A3 gene expression and upregulation of AtPP2-A8 by ABA treatment suggest that those genes are also involved in the response to abiotic stresses.
Role of AtPP2-A3 and AtPP2-A8 in defense response to H. schachtii
During nematode migration through the rhizodermis and cortex, individual cells within reach of the nematode stylet, including the endodermal cells (the innermost layer of cortex) surrounding the vascular cylinder, become damaged (Holbein et al. 2019). Reaching the vascular cylinder, sedentary cyst-forming plant-parasitic nematodes select a single parenchymatous cell as the initial syncytial cell and inject into it a mixture of effectors that modify host cell metabolism through direct or indirect interaction with host cell proteins. Resistant plants possess PTI or ETI mechanisms to prevent nematode development; susceptible plants trigger inefficient defense pathways to defend themselves (Goverse and Mitchum 2022). To achieve proper syncytium development, nematodes fine tune the expression of different plant genes, and vice versa, plants also modulate expression of their genes participating in the defense response.
Host proteins involved in plant defense responses possess characteristic NBS and LRR domains and N-terminal TIR or CC domains. The AtPP2-A3 and AtPP2-A8 genes analyzed herein encode putative lectin domains and domains present in R proteins: AIG1 and TIR, respectively (Burch-Smith and Dinesh-Kumar 2007; Whitham et al. 1994; Reuber and Ausubel 1996). The presence of these characteristic R protein domains in the proteins encoded by the AtPP2-A3 and AtPP2-A8 genes, the reduction in their expression levels during nematode attack, and the alteration of gene expression affecting the susceptibility of plants indicate the involvement of AtPP2-A3 and AtPP2-A8 in the plant's immune system.
For comparison, the previously described AtPP2-A1 protein does not possess any protein–protein interaction motifs, such as the AIG1-type G domain, TOLL, or F-Box domains located in the N-terminal regions of some other Arabidopsis PP2-like proteins (Dinant et al. 2003). It was also shown that AtPP2-A1 binds to several phloem sap proteins and may play different functions in the trafficking of endogenous proteins and in interactions with phloem-sucking insects (Beneteau et al. 2010). AtPP2-A1 did not reduce the number of pea aphids (Acyrthosiphon pisum) or green peach aphids (Myzus persicae); however, adding the recombinant PP2-A1 protein to the aphid diet caused a delay in weight gain of the nymphs (Beneteau et al. 2010). Overexpression of the AtPP2-A1 gene caused repression of M. persicae phloem-feeding activities due to molecular interaction between Mp1 (effector aphid salivary protein) and AtPP2-A1 (Wang et al. 2021), which led to reduced insect colonization (Zhang et al. 2011). In contrast, upregulation of AtPP2-A1 after pathogen attack and antifungal activity of its protein have been described (Lee et al. 2014). Overexpression of the AtPP2-B10 gene encoding the F-Box domain, expression of which is elevated after plant infection with the virulent Pseudomonas syringae pv. tomato strain DC3000 (Pst DC3000), resulted in reduced leaf damage at infection sites; in contrast to infected mutant and wild-type plants, it also caused a reduction in bacterial numbers and accumulation of anthocyanins (Stefanowicz et al. 2016; Romero-Pérez et al. 2021). Other Nictaba orthologs, such as AN3 (AtPP2-A2), AN4 (AtPP2-A9), and AN5 (AtPP2-A1), of Arabidopsis encode only the lectin domain, for which participation in the defense response against bacterial pathogens was confirmed. Overexpression of AN4(AtPP2-A9) and AN5(AtPP2-A1) significantly improves tolerance to P. syringae compared to wild-type plants (Eggermont et al. 2018). It was also shown that the AtPP2-A5 gene, encoding a protein containing lectin and TIR1 domains, confers tolerance to herbivorous two-spotted spider mites (Tetranychus urticae) through modulation of phytohormonal signaling. Overexpression or mutation of the PP2-A5 gene results in transcriptional reprogramming that modulates the balance of hormone accumulation and corresponding signaling pathways (Santamaría et al. 2019).
The role and localization of PP2-like protein activity varies depending on the amino acid sequence. In our work, we showed that modification of expression levels of the AtPP2-A3 and AtPP2-A8 genes via mutation or overexpression induced divergent responses to attack by cyst-forming nematodes. Mutations in the AtPP2-A3 and AtPP2-A8 genes led to a decrease in plant susceptibility to H. schachtii, whereas their overexpression had the opposite effect, namely, an increase in the susceptibility of transgenic Arabidopsis plants. Moreover, modulating AtPP2-A3 and AtPP2-A8 gene expression levels in mutant or overexpressing lines had a greater impact on the numbers of females than males and a relatively weaker effect on juveniles. This result suggests that these genes mainly act at later stages of nematode development and are putatively nematode sex dependent. Differential gene expression depending on whether it is a female- or male-associated syncytium has been demonstrated previously (Anjam et al. 2020).
AtPP2-A3 and AtPP2-A8 are unique genes that are downregulated by the host during nematode infestation to decrease plant susceptibility. Most likely, these genes act as negative regulators of defense pathways under regular conditions and are downregulated after pathogen attack in Arabidopsis roots to switch on the plant defense response. Sedentary plant-parasitic nematodes are able to manipulate the metabolism and gene expression of host plants to develop a specialized feeding structure, resulting in crop yield reduction. One of the solutions to this agricultural problem is plant resistance breeding. Unfortunately, the number of known and available R genes is limited. Therefore, for over 2 decades, scientists have focused on identifying and analyzing susceptibility (S) genes. S genes can be modified by genome editing tools and be used to obtain plants with increased tolerance or resistance. Unfortunately, S genes often show a pleiotropic effect, and their damage can negatively affect important physiological processes. Therefore, research in the field of functional genomics, to which we can add our work, increases the possibility of collecting gene pools to gain double or triple mutants that can be used to obtain cultivars with increased tolerance to nematodes. However, further investigation of the AtPP2-A3 and AtPP2-A8 genes to explain their function and interactions with other potential components of the plant defense system is required to make progress in understanding their mode of action.
Author contribution statement
AW developed the concept and designed the experiments, analyzed the results, performed the statistical analyses, genotyped the mutants, conducted the infection tests, and participated in and coordinated all the molecular analyses. KW and KK performed the mutant and transformant genotyping. KW, KK, and TK prepared the gene constructs and transformed plants. ER and MS performed the infection tests and microscopic analyses. KW performed the gene expression analyses. AW and MS wrote the manuscript. All authors discussed the results and commented on the manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- dpi:
-
Days post-infection
- J2:
-
Infective juveniles (J2 stage)
- Nictaba:
-
Nicotiana tabacum L. agglutinins (lectins)
- OE:
-
Overexpressing
- PP:
-
Phloem protein
References
Anjam MS, Shah SJ, Matera C et al (2020) Host factors influence the sex of nematodes parasitizing roots of Arabidopsis thaliana. Plant Cell Environ 43:1160–1174. https://doi.org/10.1111/pce.13728
Arya P, Acharya V (2018) Plant STAND P-loop NTPases: a current perspective of genome distribution, evolution, and function. Mol Genet Genom 293:17–31. https://doi.org/10.1007/s00438-017-1368-3
Beneteau J, Renard D, Marché L et al (2010) Binding properties of the N-acetylglucosamine and high-mannose N-glycan PP2-A1 phloem lectin in Arabidopsis. Plant Physiol 153:1345–1361. https://doi.org/10.1104/pp.110.153882
Burch-Smith TM, Dinesh-Kumar SP (2007) The functions of plant TIR domains. Sci STKE. https://doi.org/10.1126/stke.4012007pe46
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743. https://doi.org/10.1046/j.1365-313x.1998.00343.x
Cronshaw J, Esau K (1967) Tubular and fibrillar components of mature and differentiating sieve elements. J Cell Biol 34:801–815. https://doi.org/10.1083/jcb.34.3.801
Czechowski T, Stitt M, Altmann T et al (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139:5–17. https://doi.org/10.1104/pp.105.063743
Dangl JL, Horvath DM, Staskawicz BJ (2013) Pivoting the plant immune system from dissection to deployment. Science (80-) 341:746–751. https://doi.org/10.1126/science.1236011
Dinant S, Clark AM, Zhu Y et al (2003) Diversity of the superfamily of phloem lectins (Phloem Protein 2) in Angiosperms. Plant Physiol 131:114–128. https://doi.org/10.1104/pp.013086
Eggermont L, Verstraeten B, Van Damme EJM (2017) Genome-wide screening for lectin motifs in Arabidopsis thaliana. Plant Genome. https://doi.org/10.3835/plantgenome2017.02.0010
Eggermont L, Stefanowicz K, Van Damme EJM (2018) Nictaba homologs from Arabidopsis thaliana are involved in plant stress responses. Front Plant Sci 8:02218. https://doi.org/10.3389/fpls.2017.02218
Gheysen G, Mitchum MG (2019) Phytoparasitic nematode control of plant hormone pathways. Plant Physiol 179:1212–1226. https://doi.org/10.1104/pp.18.01067
Golinowski W, Grundler FMW, Sobczak M (1996) Changes in the structure of Arabidopsis thaliana during female development of the plant-parasitic nematode Heterodera schachtii. Protoplasma 194:103–116. https://doi.org/10.1007/BF01273172
Goverse A, Mitchum MG (2022) At the molecular plant–nematode interface: new players and emerging paradigms. Curr Opin Plant Biol 67:102225. https://doi.org/10.1016/j.pbi.2022.102225
Graeber K, Linkies A, Wood ATA, Leubner-Metzger G (2011) A guideline to family-wide comparative state-of-the-art quantitative RT-PCR analysis exemplified with a Brassicaceae cross-species seed germination case study. Plant Cell 23:2045–2063. https://doi.org/10.1105/tpc.111.084103
Holbein J, Franke RB, Marhavý P et al (2019) Root endodermal barrier system contributes to defence against plant-parasitic cyst and root-knot nematodes. Plant J 100:221–236. https://doi.org/10.1111/tpj.14459
Jones JT, Haegeman A, Danchin EGJ et al (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961. https://doi.org/10.1111/mpp.12057
Lee JR, Boltz KA, Lee SY (2014) Molecular chaperone function of Arabidopsis thaliana phloem protein 2–A1, encodes a protein similar to phloem lectin. Biochem Biophys Res Commun 443:18–21. https://doi.org/10.1016/j.bbrc.2013.11.034
Meyers BC, Kozik A, Griego A et al (2003) Genome-wide analysis of NBS-LRR–encoding genes in Arabidopsis. Plant Cell 15:809–834. https://doi.org/10.1105/tpc.009308
Nürnberger T, Lipka V (2005) Non-host resistance in plants: new insights into an old phenomenon. Mol Plant Pathol 6:335–345. https://doi.org/10.1111/j.1364-3703.2005.00279.x
Reuber TL, Ausubel FM (1996) Isolation of Arabidopsis genes that differentiate between resistance responses mediated by the RPS2 and RPM1 disease resistance genes. Plant Cell 8:241–249. https://doi.org/10.1105/tpc.8.2.241
Romero-Pérez A, Ameye M, Audenaert K, Van Damme EJM (2021) Overexpression of F-Box Nictaba promotes defense and anthocyanin accumulation in Arabidopsis thaliana after Pseudomonas syringae infection. Front Plant Sci 12:692606. https://doi.org/10.3389/fpls.2021.692606
Santamaría ME, Martínez M, Arnaiz A et al (2019) An Arabidopsis TIR-Lectin two-domain protein confers defense properties against Tetranychus urticae. Plant Physiol 179:1298–1314. https://doi.org/10.1104/pp.18.00951
Sijmons PC, Grundler FMW, von Mende N et al (1991) Arabidopsis thaliana as a new model host for plant-parasitic nematodes. Plant J 1:245–254. https://doi.org/10.1111/j.1365-313X.1991.00245.x
Sobczak M, Avrova A, Jupowicz J et al (2005) Characterization of susceptibility and resistance responses to potato cyst nematode (Globodera spp.) infection of tomato lines in the absence and presence of the broad-spectrum nematode resistance Hero gene. Mol Plant-Microbe Interact 18:158–168. https://doi.org/10.1094/MPMI-18-0158
Stefanowicz K, Lannoo N, Zhao Y et al (2016) Glycan-binding F-box protein from Arabidopsis thaliana protects plants from Pseudomonas syringae infection. BMC Plant Biol 16:213. https://doi.org/10.1186/s12870-016-0905-2
Szakasits D, Heinen P, Wieczorek K et al (2009) The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. Plant J 57:771–784. https://doi.org/10.1111/j.1365-313X.2008.03727.x
Varypatakis K, Véronneau P-Y, Thorpe P, Cock PJA, Lim JT-Y, Armstrong MR, Janakowski S, Sobczak M, Hein I, Mimee B, Jones JT, Blok VC (2020) The genomic impact of selection for virulence against resistance in the potato cyst nematode, Globodera pallida. Genes 11:1429. https://doi.org/10.3390/genes11121429
Van Der Biezen EA, Jones JDG (1998) Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem Sci 23:454–456. https://doi.org/10.1016/S0968-0004(98)01311-5
Wang Z, Lü Q, Zhang L et al (2021) Aphid salivary protein Mp1 facilitates infestation by binding phloem protein 2–A1 in Arabidopsis. Biochem Biophys Res Commun 572:105–111. https://doi.org/10.1016/j.bbrc.2021.07.066
Whitham S, Dinesh-Kumar SP, Choi D et al (1994) The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell 78:1101–1115. https://doi.org/10.1016/0092-8674(94)90283-6
Wiśniewska A, Dąbrowska-Bronk J, Szafrański K et al (2013) Analysis of tomato gene promoters activated in syncytia induced in tomato and potato hairy roots by Globodera rostochiensis. Transgenic Res 22:557–569. https://doi.org/10.1007/s11248-012-9665-4
Wiśniewska A, Wojszko K, Różańska E et al (2021) Arabidopsis thaliana Myb59 gene is involved in the response to Heterodera schachtii infestation, and its overexpression disturbs regular development of nematode-induced syncytia. Int J Mol Sci 22:6450. https://doi.org/10.3390/ijms22126450
Wiśniewska A, Wojszko K, Różańska E et al (2022) Arabidopsis thaliana AtHRS1 gene is involved in the response to Heterodera schachtii infection and its overexpression hampers development of syncytia and involves a jasmonic acid-dependent mechanism. J Plant Physiol 272:153680. https://doi.org/10.1016/j.jplph.2022.153680
Zhang C, Shi H, Chen L et al (2011) Harpin-induced expression and transgenic overexpression of the phloem protein gene AtPP2-A1 in Arabidopsis repress phloem feeding of the green peach aphid Myzus persicae. BMC Plant Biol 11:11. https://doi.org/10.1186/1471-2229-11-11
Funding
This work was financed by the Polish National Science Centre (NCN) on the basis of the decision number DEC-2015/17/B/NZ9/01767 (granted to Anita Wiśniewska).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no significant competing financial, professional, or personal interests that might have influenced the performance or presentation of the work described in this manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wojszko, K., Różańska, E., Sobczak, M. et al. The role of AtPP2-A3 and AtPP2-A8 genes encoding Nictaba-related lectin domains in the defense response of Arabidopsis thaliana to Heterodera schachtii. Planta 258, 40 (2023). https://doi.org/10.1007/s00425-023-04196-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-023-04196-y