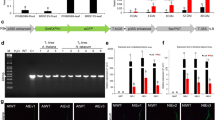
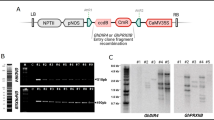
Abstract
Main conclusion
The overexpression of the GmGlb1-1 gene reduces plant susceptibility to Meloidogyne incognita.
Abstract
Non-symbiotic globin class #1 (Glb1) genes are expressed in different plant organs, have a high affinity for oxygen, and are related to nitric oxide (NO) turnover. Previous studies showed that soybean Glb1 genes are upregulated in soybean plants under flooding conditions. Herein, the GmGlb1-1 gene was identified in soybean as being upregulated in the nematode-resistant genotype PI595099 compared to the nematode-susceptible cultivar BRS133 during plant parasitism by Meloidogyne incognita. The Arabidopsis thaliana and Nicotiana tabacum transgenic lines overexpressing the GmGlb1-1 gene showed reduced susceptibility to M. incognita. Consistently, gall morphology data indicated that pJ2 nematodes that infected the transgenic lines showed developmental alterations and delayed parasitism progress. Although no significant changes in biomass and seed yield were detected, the transgenic lines showed an elongated, etiolation-like growth under well-irrigation, and also developed more axillary roots under flooding conditions. In addition, transgenic lines showed upregulation of some important genes involved in plant defense response to oxidative stress. In agreement, higher hydrogen peroxide accumulation and reduced activity of reactive oxygen species (ROS) detoxification enzymes were also observed in these transgenic lines. Thus, based on our data and previous studies, it was hypothesized that constitutive overexpression of the GmGlb1-1 gene can interfere in the dynamics of ROS production and NO scavenging, enhancing the acquired systemic acclimation to biotic and abiotic stresses, and improving the cellular homeostasis. Therefore, these collective data suggest that ectopic or nematode-induced overexpression, or enhanced expression of the GmGlb1-1 gene using CRISPR/dCas9 offers great potential for application in commercial soybean cultivars aiming to reduce plant susceptibility to M. incognita.





Similar content being viewed by others
Data availability statement
The partial genome sequences are provided in the NCBI database from GenBank accession number: GmGlb1.1_PI595099: ON228174 and GmGlb1-1_BRS133: ON228175. The nucleotide sequence can be accessed at https://www.ncbi.nlm.nih.gov/nuccore. The Sequence Read Archive (SRA) data from RNAseq are provided in the NCBI database from BioProject number: PRJNA75066. The BioProject can be accessed at https://www.ncbi.nlm.nih.gov/bioproject/. In addition, genome target sequence and transcriptome data, such as gene expression, are also provided by authors as supplementary data.
Abbreviations
- DAI:
-
Days after inoculation
- Glb:
-
Globin
- J2:
-
Second-stage juveniles
- NO:
-
Nitric oxide
- pJ2:
-
Parasitic second-stage juveniles
- ppJ2:
-
Pre-parasitic second-stage juveniles
- Ev:
-
Transgenic event
References
Abad P, Gouzy J, Aury J-M, Castagnone-Sereno P, Danchin EGJ, Deleury E, Perfus-Barbeoch L, Anthouard V, Artiguenave F, Blok VC, Caillaud M-C, Coutinho PM, Dasilva C, De Luca F, Deau F, Esquibet M, Flutre T, Goldstone JV, Hamamouch N, Hewezi T, Jaillon O, Jubin C, Leonetti P, Magliano M, Maier TR, Markov GV, McVeigh P, Pesole G, Poulain J, Robinson-Rechavi M, Sallet E, Ségurens B, Steinbach D, Tytgat T, Ugarte E, van Ghelder C, Veronico P, Baum TJ, Blaxter M, Bleve-Zacheo T, Davis EL, Ewbank JJ, Favery B, Grenier E, Henrissat B, Jones JT, Laudet V, Maule AG, Quesneville H, Rosso M-N, Schiex T, Smant G, Weissenbach J, Wincker P (2008) Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol 26:909
Ali MA, Azeem F, Abbas A, Joyia FA, Li H, Dababat AA (2017) Transgenic strategies for enhancement of nematode resistance in plants. Front Plant Sci 8:750
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Arthikala MK, Montiel J, Sánchez-López R, Nava N, Cárdenas L, Quinto C (2017) Respiratory rurst oxidase homolog gene A is crucial for rhizobium infection and nodule maturation and function in common bean. Front Plant Sci 8:2003
Barata RM, Chaparro A, Chabregas SM, González R, Labate CA, Azevedo RA, Sarath G, Lea PJ, Silva-Filho MC (2000) Targeting of the soybean leghemoglobin to tobacco chloroplasts: effects on aerobic metabolism in transgenic plants. Plant Sci 155:193–202
Basso MF, Ferreira PCG, Kobayashi AK, Harmon FG, Nepomuceno AL, Molinari HBC, Grossi-de-Sa MF (2019) MicroRNAs and new biotechnological tools for its modulation and improving stress tolerance in plants. Plant Biotechnol J 17:1482–1500
Basso MF, Arraes FBM, Grossi-de-Sa M, Moreira VJV, Alves-Ferreira M, Grossi-de-Sa MF (2020a) Insights into genetic and molecular elements for transgenic crop development. Front Plant Sci 11:509
Basso MF, Lourenço-Tessutti IT, Mendes RAG, Pinto CEM, Bournaud C, Gillet F-X, Togawa RC, de Macedo LLP, Engler JA, Grossi-de-Sa MF (2020b) MiDaf16-like and MiSkn1-like gene families are reliable targets to develop biotechnological tools for the control and management of Meloidogyne incognita. Sci Rep 10:6991
Bellafiore S, Shen Z, Rosso M-N, Abad P, Shih P, Briggs SP (2008) Direct identification of the Meloidogyne incognita secretome reveals proteins with host cell reprogramming potential. PLoS Pathog 4:e1000192
Bernard GC, Egnin M, Bonsi C (2017) The impact of plant-parasitic nematodes on agriculture and methods of control. In: Shah MM, Mahamood MN (eds) Nematology - concepts, diagnosis and control. IntechOpen, London. https://doi.org/10.5772/intechopen.68958
Bonna AL, Chaparro-Giraldo A, Appezzato-da-Gloria B, Hedden P, Silva-Filho MC (2008) Ectopic expression of soybean leghemoglobin in chloroplasts impairs gibberellin biosynthesis and induces dwarfism in transgenic potato plants. Mol Breed 22:613–618
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bülow L, Holmberg N, Lilius G, Bailey JE (1999) The metabolic effects of native and transgenic hemoglobins on plants. Trends Biotechnol 17:21–24
Carneiro RG, Mazzafera P, Ferraz LCCB, Muraoka T, Trivelin PCO (2002) Uptake and translocation of nitrogen, phosphorus and calcium in soybean infected with Meloidogyne incognita and M. javanica. Fitopatol Bras 27:141–150
Castagnone-Sereno P, Danchin EGJ, Perfus-Barbeoch L, Abad P (2013) Diversity and evolution of root-knot nematodes, genus Meloidogyne: new insights from the genomic Era. Annu Rev Phytopathol 51:203–220
Chaparro-Giraldo A, Barata RM, Chabregas SM, Azevedo RA, Silva-Filho MC (2000) Soybean leghemoglobin targeted to potato chloroplasts influences growth and development of transgenic plants. Plant Cell Rep 19:961–965
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant Journal 16(6):735–743. https://doi.org/10.1046/j.1365-313x.1998.00343.x
Cosio C, Ranocha P, Francoz E, Burlat V, Zheng Y, Perry SE, Ripoll J-J, Yanofsky M, Dunand C (2017) The class III peroxidase PRX17 is a direct target of the MADS-box transcription factor AGAMOUS-LIKE15 (AGL15) and participates in lignified tissue formation. New Phytol 213:250–263
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36:W465-469
Dieryck W, Pagnier J, Poyart C, Marden MC, Gruber V, Bournat P, Baudino S, Mérot B (1997) Human haemoglobin from transgenic tobacco. Nature 386:29–30
Dmitryukova MY, Baimiev AK, Fedyaev VV, Rakhmankulova ZF (2011) Effect of leghemoglobin A gene expression from soybean on tobacco plant growth and antioxidant state under damaging action of cadmium. Russ J Plant Physiol 58:1055
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2012) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21
Engler JA, Vieira P, Rodiuc N, Grossi de Sa MF, Engler G (2015) Chapter four—the plant cell cycle machinery: usurped and modulated by plant-parasitic nematodes. Adv Bot Res 73:91–118 (Escobar C, Fenoll C (eds))
Farnese FS, Menezes-Silva PE, Gusman GS, Oliveira JA (2016) When bad guys become good ones: the key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front Plant Sci 7:471
Fragoso RR, Arraes FBM, Lourenço-Tristan I, Miranda VJ, Basso MF, Ferreira AVJ, Viana AAB, Lins CBJ, Lins PC, Engler GJ, Moura SM, Batista JAN, Silva MCM, Engler G, Morgante CV, Lisei-de-Sa ME, Vasques RM, Engler JA, Grossi-de-Sa MF (2022) Functional characterization of the pUceS8.3 promoter and its potential use for ectopic gene overexpression. PLANTA 256:69. https://doi.org/10.1007/s00425-022-03980-6
Garrocho-Villegas V, Gopalasubramaniam SK, Arredondo-Peter R (2007) Plant hemoglobins: what we know six decades after their discovery. Gene 398:78–85
Gechev TS, Hille J (2005) Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol 168:17–20
Gillet FX, Bournaud C, Junior JDAS, Grossi-de-Sa MF (2017) Plant-parasitic nematodes: towards understanding molecular players in stress responses. Ann Bot 119:775–789
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40:D1178–D1186
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652
Gupta KJ, Igamberdiev AU (2016) Reactive nitrogen species in mitochondria and their implications in plant energy status and hypoxic stress tolerance. Front Plant Sci 7:369
Hamawaki OT, Hamawaki RL, Nogueira APO, Glasenapp JS, Hamawaki CDL, Silva COD (2019) Evaluation of soybean breeding lineages to new sources of root-knot nematode resistance. Cienc Agrotec 43:e009519
Hartman GL, West ED, Herman TK (2011) Crops that feed the world 2. Soybean-worldwide production, use, and constraints caused by pathogens and pests. Food Secur 3:5–17
Hasan MS, Singh V, Islam S, Islam MS, Ahsan R, Kaundal A, Islam T, Ghosh A (2021) Genome-wide identification and expression profiling of glutathione S-transferase family under multiple abiotic and biotic stresses in Medicago truncatula L. PLoS ONE 16:e0247170
Hebelstrup KH, Jensen EØ (2008) Expression of NO scavenging hemoglobin is involved in the timing of bolting in Arabidopsis thaliana. Planta 227:917–927
Hebelstrup KH, Hunt P, Dennis E, Jensen SB, Jensen EO (2006) Hemoglobin is essential for normal growth of Arabidopsis organs. Physiol Plant 127:157–166
Hill RD (2012) Non-symbiotic haemoglobins—what’s happening beyond nitric oxide scavenging? AoB Plants 2012:pls004
Holmberg N, Lilius G, Bailey JE, Bülow L (1997) Transgenic tobacco expressing Vitreoscilla hemoglobin exhibits enhanced growth and altered metabolite production. Nat Biotechnol 15:244–247
Hussey RS, Barker KR (1973) A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis Rep 57:1025–1028
Igamberdiev AU, Hill RD (2004) Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. J Exp Bot 55:2473–2482
Junior JDAS, Pierre O, Coelho RR, Grossi-de-Sa MF, Engler G, Engler JA (2017) Application of nuclear volume measurements to comprehend the cell cycle in root-knot nematode-induced giant cells. Front Plant Sci 8:961
Koltun A, Fuhrmann-Aoyagi MB, Moraes LAC, Nepomuceno AL, Gonçalves LSA, Mertz-Henning LM (2021) Uncovering the roles of hemoglobins in soybean facing water stress. Gene 810:146055
Koutsovoulos GD, Marques E, Arguel M-J, Machado AC, Carneiro RMDG, Castagnone-Sereno P, Danchin EG, Albuquerque EV (2018) Parallel adaptations to different host plants despite clonal reproduction in the world most devastating nematode pest. BioRxiv 362129
Labudda M, Różańska E, Gietler M, Fidler J, Muszyńska E, Prabucka B, Morkunas I (2020) Cyst nematode infection elicits alteration in the level of reactive nitrogen species, protein s-nitrosylation and nitration, and nitrosoglutathione reductase in Arabidopsis thaliana roots. Antioxidants (basel) 9:795
Lu P, Davis RF, Kemerait RC, van Iersel MW, Scherm H (2014) Physiological effects of Meloidogyne incognita infection on cotton genotypes with differing levels of resistance in the greenhouse. J Nematol 46:352–359
Ma L, Shi Y, Siemianowski O, Yuan B, Egner TK, Mirnezami SV, Lind KR, Ganapathysubramanian B, Venditti V, Cademartiri L (2019) Hydrogel-based transparent soils for root phenotyping in vivo. Proc Natl Acad Sci USA 116:11063–11068
Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Geer LY, Bryant SH (2017) CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res 45:D200–D203
Mejias J, Truong NM, Abad P, Favery B, Quentin M (2019) Plant proteins and processes targeted by parasitic nematode effectors. Front Plant Sci 10:970
Mendes RA, Basso MF, Fernandes-de-Araújo J, Paes-de-Melo B, Lima RN, Ribeiro TP, da Silva-Mattos V, Saliba-Albuquerque EV, Grossi-de-Sa M, Dessaune-Tameirao SN, da Rocha-Fragoso R, Mattar-da-Silva MC, Vignols F, Fernandez D, Grossi-de-Sa MF (2021a) Minc00344 and Mj-NULG1a effectors interact with GmHub10 protein to promote the soybean parasitism by Meloidogyne incognita and M javanica. Exp Parasitol 229:108153
Mendes RA, Basso MF, Paes de Melo B, Ribeiro TP, Lima RN, Fernandes de Araújo J, Grossi-de-Sa M, da Silva MV, Togawa RC, Saliba Albuquerque ÉV, Lisei-de-Sa ME, Mattar da Silva MC, Pepino Macedo LL, da Rocha FR, Fernandez D, Vignols F, Grossi-de-Sa MF (2021b) The Mi-EFF1/Minc17998 effector interacts with the soybean GmHub6 protein to promote host plant parasitism by Meloidogyne incognita. Physiol Mol Plant Pathol 114:101630
Muhire BM, Varsani A, Martin DP (2014) SDT: a virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE 9:e108277
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakayama TJ, Rodrigues FA, Neumaier N, Marcolino-Gomes J, Molinari HBC, Santiago TR, Formighieri EF, Basso MF, Farias JRB, Emygdio BM, de Oliveira ACB, Campos AD, Borém A, Harmon FG, Mertz-Henning LM, Nepomuceno AL (2017) Insights into soybean transcriptome reconfiguration under hypoxic stress: functional, regulatory, structural, and compositional characterization. PLoS ONE 12:e0187920
Neuser J, Metzen CC, Dreyer BH, Feulner C, van Dongen JT, Schmidt RR, Schippers JHM (2019) HBI1 mediates the trade-off between growth and immunity through its impact on apoplastic ROS homeostasis. Cell Rep 28:1670–1678
Oliveira JTA, Araujo-Filho JH, Grangeiro TB, Gondim DMF, Segalin J, Pinto PM, Carlini CRRS, Silva FDA, Lobo MDP, Costa JH, Vasconcelos IM (2014) Enhanced synthesis of antioxidant enzymes, defense proteins and leghemoglobin in rhizobium-free cowpea roots after challenging with Meloydogine incognita. Proteomes 2:527–549
Oostenbrink M (1966) Major characteristics of the relation between nematode and plants. Meded Landbouwhogeschool 66:3–46
Paes-de-Melo B, Lourenço-Tessutti IT, Fraga OT, Pinheiro LB, de Jesus Lins CB, Morgante CV, Engler JA, Reis PAB, Grossi-de-Sá MF, Fontes EPB (2021) Contrasting roles of GmNAC065 and GmNAC085 in natural senescence, plant development, multiple stresses and cell death responses. Sci Rep 11(1):11178
Park SH, Rose SC, Zapata C, Srivatanakul M, Smith RH (1998) Cross-protection and selectable marker genes in plant transformation. In Vitro Cell Dev Biol Plant 34:117–121
Perazzolli M, Dominici P, Romero-Puertas MC, Zago E, Zeier J, Sonoda M, Lamb C, Delledonne M (2004) Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell 16:2785–2794
Sato K, Kadota Y, Shirasu K (2019) Plant immune responses to parasitic nematodes. Front Plant Sci 10:1165
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101
Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, Xu D, Hellsten U, May GD, Yu Y, Sakurai T, Umezawa T, Bhattacharyya MK, Sandhu D, Valliyodan B, Lindquist E, Peto M, Grant D, Shu S, Goodstein D, Barry K, Futrell-Griggs M, Abernathy B, Du J, Tian Z, Zhu L, Gill N, Joshi T, Libault M, Sethuraman A, Zhang XC, Shinozaki K, Nguyen HT, Wing RA, Cregan P, Specht J, Grimwood J, Rokhsar D, Stacey G, Shoemaker RC, Jackson SA (2010) Genome sequence of the palaeopolyploid soybean. Nature 463:178–183
Seo Y, Kim YH (2014) Control of Meloidogyne incognita using mixtures of organic acids. Plant Pathol J 30:450–455
Shukla N, Yadav R, Kaur P, Rasmussen S, Goel S, Agarwal M, Jagannath A, Gupta R, Kumar A (2018) Transcriptome analysis of root-knot nematode (Meloidogyne incognita)-infected tomato (Solanum lycopersicum) roots reveals complex gene expression profiles and metabolic networks of both host and nematode during susceptible and resistance responses. Mol Plant Pathol 19:615–633
Singh S, Varma A (2017) Structure, function, and estimation of leghemoglobin. In: Hansen AP, Choudhary DK, Agrawal PK, Varma A (eds) Rhizobium biology and biotechnology. Springer International Publishing, Cham, pp 309–330. https://doi.org/10.1007/978-3-319-64982-5_15
Stothard P (2000) The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 28:1102–1104
Tang Y, Liu Q, Liu Y, Zhang L, Ding W (2017) Overexpression of NtPR-Q up-regulates multiple defense-related genes in Nicotiana tabacum and enhances plant resistance to Ralstonia solanacearum. Front Plant Sci 8:1963
Trudgill DL, Blok VC (2001) Apomictic, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annu Rev Phytopathol 39:53–77
Tylka GL, Marett CC (2021) Known distribution of the soybean cyst nematode, Heterodera glycines, in the United States and Canada in 2020. Plant Health Prog 22:72–74
Valliyodan B, Van Toai TT, Alves JD, Goulart PFP, Lee JD, Fritschi FB, Rahman MA, Islam R, Shannon JG, Nguyen HT (2014) Expression of root-related transcription factors associated with flooding tolerance of soybean (Glycine max). Int J Mol Sc 15:17622–17643
Windham GL, Williams WP (1987) Host suitability of commercial corn hybrids to Meloidogyne arenaria and M. incognita. J Nematol 19:13–16
Zhou J, Jia F, Shao S, Zhang H, Li G, Xia X, Zhou Y, Yu J, Shi K (2015) Involvement of nitric oxide in the jasmonate-dependent basal defense against root-knot nematode in tomato plants. Front Plant Sci 6:193–193
Acknowledgements
We are grateful to EMBRAPA, CAPES, CNPq, FAPESP, INCT PlantStress Biotech, INCT Bioethanol, and FAP-DF for the scientific research support. Marcela Araújo Santos, Eglee Silvia Gonçalves Igarashi, and Viviane Lopes da Costa for providing technical support in the lab.
Funding
MFB is grateful to CAPES for the postdoctoral research fellowship (process number: 88887.642997/2021-–00). AG is grateful to FAPESP 2019/13936-0. ITLT and CMP are grateful to CAPES/Cofecub project for financial support in the researcher and students exchange program between institutions. MFGS is grateful for grants from CNPq, FAP-DF, INCT Plant Stress Biotech, CAPES, and EMBRAPA. MSB is grateful for grants from INCT Bioethanol and FAPESP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent for publication or ethical approval and consent to participate
Not applicable.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
425_2022_3992_MOESM1_ESM.tif
Suppl. Fig. S1 Identification of orthologous genes of GmGlb1-1 (Glyma.11G121800) in genomes of nematode-resistant genotype PI595099 and nematode-susceptible cultivar BRS133. Pairwise sequence identity matrix from a transcript, b CDS, and c amino acid sequences of the GmGlb1-1 gene. d Alignment of amino acid sequences of the GmGlb1-1 gene, which were retrieved from genome sequencing datasets generated from genotype PI595099 and cultivar BRS133, and Glycine max Wm82.a2.v1 (BioProject: PRJNA19861). e and f In silico analysis of soybean GmGlb1-1 gene. The GmGlb1-1 (Glyma.11G121800) gene used in our study is highlighted in the red box. Positional conservation of globin-like superfamily domain (class1_nsHb_like cd:14784) generated from multiple sequence alignment by Color Align Conservation software while sequence alignment was performed using MEGA X 10 software. g Heatmap of expression profiles (Log2 of the recorded FPKM) of the soybean Globin genes in different plant tissues. The expression data were retrieved from the JGI Plant Gene Atlas Project available in Phytozome v.13 database, while data were then analyzed and viewed using Phytomine software (http://phytozome.jgi.doe.gov/phytomine/). Hierarchical clustering was performed for the transcript ratios from all conditions. The color scale shown below represents gene expression values, with blue indicating low levels while green indicating high levels of transcript abundance. FPKM values were calculated from a cufflink’s analysis of the aligned RNAseq data. h Correlation analysis from expression profiles (Log2 of the recorded FPKM) of soybean Globin genes was analyzed and viewed using Phytomine software. The globin sequences from soybean (Glycine max), bean (Phaseolus vulgaris), red clover (Trifolium pretense), barrel clover (Medicago truncatula), Arabidopsis (Arabidopsis thaliana), tomato (Solanum lycopersicum), cotton (Gossypium hirsutum), and cowpea (Vigna unguiculata) were retrieved of the Glycine max Wm82.a2.v1 (BioProject: PRJNA19861) dataset from Phytozome v.13 database (TIF 3232 KB)
425_2022_3992_MOESM2_ESM.tif
Suppl. Fig. S2 Sequence analysis of soybean GmGlb1-1 gene. The GmGlb1-1 (Glyma.11G121800) gene used in our study was highlighted with the red box. Pairwise sequence identity matrix from nucleotide a and amino acid b sequences generated using the Sequence Demarcation Tool Version 1.2 software. Evolutionary analysis from nucleotide c and e and amino acid d and f sequences generated from Phylogeny.fr web service. The globin sequences from Glycine max, Phaseolus vulgaris, Trifolium pretense, Medicago truncatula, Arabidopsis thaliana, Solanum lycopersicum, Gossypium hirsutum, and Vigna unguiculata were retrieved of the Glycine max Wm82.a2.v1 (BioProject: PRJNA19861) dataset from Phytozome v.13 database (TIF 2078 KB)
425_2022_3992_MOESM3_ESM.tif
Suppl. Fig. S3 GmGlb1-1 gene expression profile measured by real-time PCR from a 12 T1 A. thaliana and b 22 T0 N. tabacum transgenic lines compared with wild-type plants. The fold-change values were calculated with the 2^-∆∆CT formula and normalized with AtActin 2 and NtActin 4 as endogenous reference genes (Suppl. Table S3). Error bars represent confidence intervals corresponding to three technical replicates. Different letters indicate significant statistical differences between different transgenic lines based on Tukey’s test at 95% significance level. (TIF 453 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Basso, M.F., Lourenço-Tessutti, I.T., Moreira-Pinto, C.E. et al. Overexpression of a soybean Globin (GmGlb1-1) gene reduces plant susceptibility to Meloidogyne incognita. Planta 256, 83 (2022). https://doi.org/10.1007/s00425-022-03992-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-022-03992-2