Abstract
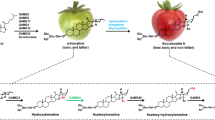
Fragaria × ananassa UDP-glucose:cinnamate glucosyltransferase (FaGT2) catalyzes the formation of cinnamic acid and p-coumaric acid glucose esters during strawberry fruit ripening. Here, the ripening and oxidative stress induced enzyme was further characterized by testing a range of structurally different substrates of natural and unnatural origin in vitro and comparing their kinetic parameters to elucidate its additional biological functions. The accepted substrates ranged from derivatives of cinnamic acid and benzoic acid to heterocyclic and aliphatic compounds resulting in the formation of O- and S-glucose esters, as well as O-glucosides. In planta assays confirmed the formation of glucose derivatives after injection of the substrates into strawberry fruits. Common chemical and structural features required for activity were the easy subtraction of a proton from the glucosylation site and the conjugation of the formed anion with π-electrons as best realized in the simplest substrate sorbic acid. In addition to cinnamic acid, the natural compounds anthranilic acid, trans-2-hexenoic acid, nicotinic acid and 2,5-dimethyl-4-hydroxy-3[2H]-furanone were glucosylated in vitro. But FaGT2 was also capable of efficiently converting xenobiotic substances like the herbicide 2,4,5-trichlorophenol and the herbicide analogue 3,5-dichloro-4-hydroxybenzoic acid. The results suggest that FaGT2 is involved in the detoxification of xenobiotics in accordance to its induction by oxidative stress.



Similar content being viewed by others
Abbreviations
- ESI:
-
Electrospray ionization
- HPLC:
-
High performance liquid chromatography
- MS:
-
Mass spectrometry
- TCP:
-
2,4,5-Trichlorophenol
- UGT:
-
UDP-glucose-dependent glucosyltransferase
- UV:
-
Ultraviolet
References
Aharoni A, Keizer LCP, Van den Broeck HC, Blanco-Portales R, oz-Blanco J, Bois G, Smit P, De Vos RCH, O’Connell AP (2002) Novel insight into vascular, stress, and auxin-dependent and -independent gene expression programs in strawberry, a non-climacteric fruit. Plant Physiol 129:1019–1031
Aubert C, Baumann S, Arguel H (2005) Optimization of the analysis of flavor volatile compounds by liquid-liquid microextraction (LLME). Application to the aroma analysis of melons, peaches, grapes, strawberries, and tomatoes. J Agric Food Chem 53:8881–8895
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Brazier-Hicks M, Edwards R (2005) Functional importance of the family 1 glucosyltransferase UGT72B1 in the metabolism of xenobiotics in Arabidopsis thaliana. Plant J 42:556–566
Ford CM, Boss PK, Hmj PB (1998) Cloning and characterization of Vitis vinifera UDP-glucose:flavonoid 3-O-glucosyltransferase, a homologue of the enzyme encoded by the maize Bronze-1 locus that may primarily serve to glucosylate anthocyanidins in vivo. J Biol Chem 273:9224–9233
Fraissinet-Tachet L, Baltz R, Chong J, Kauffmann S, Fritig B, Saindrenan P (1998) Two tobacco genes induced by infection, elicitor and salicylic acid encode glucosyltransferases acting on phenylpropanoids and benzoic acid derivatives, including salicylic acid. FEBS Lett 437:319–323
Gomes Da Silva MDR, Chaves Das Neves HJ (1999) Complementary use of hyphenated purge-and-trap gas chromatography techniques and sensory analysis in the aroma profiling of strawberries (Fragaria ananassa). J Agric Food Chem 47:4568–4573
Groyne J, Lognay G, Marlier M (1999) Accumulation of glycosidically bound compounds in Fragaria × ananassa cv. Elsanta fruits at various developmental stages. Biotechnol Agron Soc Environ 3:5–9
Hefner T, Arend J, Warzecha H, Siems K, Stöckigt J (2002) Arbutin synthase, a novel member of the NRD1β-glycosyltransferase family, is a unique multifunctional enzyme converting various natural products and xenobiotics. Bioorgan Med Chem 10:1731–1741
Jackson RG, Lim EK, Li Y, Kowalczyk M, Sandberg G, Hogget J, Ashford DA, Bowles DJ (2001) Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. J Biol Chem 276:4350–4356
Jones P, Vogt T (2001) Glycosyltransferases in secondary plant metabolism: Tranquilizers and stimulant controllers. Planta 213:164–174
Jones PR, Møller BL, Hoj PB (1999) The UDP-glucose:p-hydroxymandelonitrile-O-glucosyltransferase that catalyzes the last step in synthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor. Isolation, cloning, heterologous expression, and substrate specificity. J Biol Chem 274:35483–35491
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48:251–275
Langlois-Meurinne M, Gachon CMM, Saindrenan P (2005) Pathogen-responsive expression of glycosyltransferase genes UGT73B3 and UGT73B5 is necessary for resistance to Pseudomonas syringae pv tomato in Arabidopsis. Plant Physiol 139:1890–1901
Latza S, Berger RG (1997) 1-O-trans-cinnamoyl-β-d-glucopyranose:alcohol cinnamoyltransferase activity in fruits of cape gooseberry (Physalis peruviana L.). Z Naturforsch C 52:747–755
Lee HI, Raskin I (1999) Purification, cloning, and expression of a pathogen inducible UDP-glucose:salicylic acid glucosyltransferase from tobacco. J Biol Chem 274:36637–36642
Lehfeldt C, Shirley AM, Meyer K, Ruegger MO, Cusumano JC, Viitanen PV, Strack D, Chapple C (2000) Cloning of the SNG1 gene of Arabidopsis reveals a role for a serine carboxypeptidase-like protein as an acyltransferase in secondary metabolism. Plant Cell 12:1295–1306
Lim EK, Li Y, Parr A, Jackson R, Ashford DA, Bowles DJ (2001) Identification of glucosyltransferase genes involved in sinapate metabolism and lignin synthesis in Arabidopsis. J Biol Chem 276:4344–4349
Lim EK, Doucet CJ, Li Y, Elias L, Worrall D, Spencer SP, Ross J, Bowles DJ (2002) The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4-hydroxybenzoic acid, and other benzoates. J Biol Chem 277:586–592
Loutre C, Dixon DP, Brazier M, Slater M, Cole DJ, Edwards R (2003) Isolation of a glucosyltransferase from Arabidopsis thaliana active in the metabolism of the persistent pollutant 3,4-dichloroaniline. Plant J 34:485–493
Lunkenbein S, Bellido M, Aharoni A, Salentijn EMJ, Kaldenhoff R, Coiner HA, Munoz-Blanco J, Schwab W (2006a) Cinnamate metabolism in ripening fruit. Characterization of a UDP-glucose:cinnamate glucosyltransferase from strawberry. Plant Physiol 140:1047–1058
Lunkenbein S, Coiner H, de Vos CHR, Schaart JG, Boone MJ, Krens FA, Schwab W, Salentijn EMJ (2006b) Molecular characterization of a stable antisense chalcone synthase phenotype in strawberry (Fragaria × ananassa). J Agric Food Chem 54:2145–2153
Marrs KA (1996) The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47:127–158
Meßner B, Thulke O, Schäffner AR (2003) Arabidopsis glucosyltransferases with activities toward both endogenous and xenobiotic substrates. Planta 217:138–146
Milkowski C, Baumert A, Schmidt D, Nehlin L, Strack D (2004) Molecular regulation of sinapate ester metabolism in Brassica napus: expression of genes, properties of the encoded proteins and correlation of enzyme activities with metabolite accumulation. Plant J 38:80–92
Pflugmacher S, Sandermann J (1998) Taxonomic distribution of plant glucosyltransferases acting on xenobiotics. Phytochemistry 49:507–511
Poppenberger B, Berthiller F, Lucyshyn D, Sieberer T, Schuhmacher R, Krska R, Kuchler K, Glössl J, Luschnig C, Adam G (2003) Detoxification of the Fusarium Mycotoxin Deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J Biol Chem 278:47905–47914
Poppenberger B, Fujioka S, Soeno K, George GL, Vaistij FE, Hiranuma S, Seto H, Takatsuto S, Adam G, Yoshida S, Bowles D (2005) The UGT73C5 of Arabidopsis thaliana glucosylates brassinosteroids. Proc Natl Acad Sci USA 102:15253–15258
Quiel JA, Bender J (2003) Glucose conjugation of anthranilate by the Arabidopsis UGT74F2 glucosyltransferase is required for tryptophan mutant blue fluorescence. J Biol Chem 278:6275–6281
Roscher R, Herderich M, Steffen JP, Schreier P, Schwab W (1996) 2,5-dimethyl-4-hydroxy-3[2H]-furanone 6’O-malonyl-β-d-glucopyranoside in strawberry fruits. Phytochemistry 43:155–159
Schaller B, Schneider B, Schütte HR (1992) Metabolism of the herbicide bromoxynil in Hordeum vulgare and Stellaria media. Z Naturforsch C 47:126–131
Taguchi G, Imura H, Maeda Y, Kodaira R, Hayashida N, Shimosaka M, Okazaki M (2000) Purification and characterization of UDP-glucose:hydroxycoumarin 7-O-glucosyltransferase, with broad substrate specificity from tobacco cultured cells. Plant Sci 157:105–112
Taguchi G, Yazawa T, Hayashida N, Okazaki M (2001) Molecular cloning and heterologous expression of novel glucosyltransferases from tobacco cultured cells that have broad substrate specificity and are induced by salicylic acid and auxin. Eur J Biochem 268:4086–4094
Ulrich D, Hoberg E, Rapp A, Kecke S (1997) Analysis of strawberry flavor. Discrimination of aroma types by quantification of volatile compounds. Z Lebensm Unters Forsch A 205:218–223
Villegas RJA, Kojima M (1986) Purification and characterization of hydroxycinnamoyl d-glucose:Quinate hydroxycinnamoyl transferase in the root of the sweet potato, Ipomoea batatas Lam. J Biol Chem 261:8729–8733
Vogt T, Jones P (2000) Glycosyltransferases in plant-natural product synthesis: characterization of a supergene family. Trends Plant Sci 5:380–386
Wang J, De Luca V (2005) The biosynthesis and regulation of biosynthesis of Concord grape fruit esters, including ‘foxy’ methylanthranilate. Plant J 44:606–619
Wintoch H, Krammer G, Schreier P (1991) Glycosidically bound aroma compounds from two strawberry fruit species, Fragaria vesca f. semperflorens and Fragaria × ananassa, cv. Korona. Flavour Frag J 6:209–215
Acknowledgments
We thank Heather Coiner for correcting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
GenBank Accession number of FaGT2: AY663785.
Rights and permissions
About this article
Cite this article
Landmann, C., Fink, B. & Schwab, W. FaGT2: a multifunctional enzyme from strawberry (Fragaria × ananassa) fruits involved in the metabolism of natural and xenobiotic compounds. Planta 226, 417–428 (2007). https://doi.org/10.1007/s00425-007-0492-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0492-4