Abstract
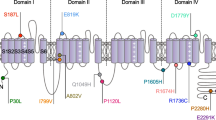
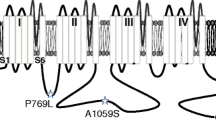
Trigeminal neuralgia is a rare and debilitating disorder that affects one or more branches of the trigeminal nerve, leading to severe pain attacks and a poor quality of life. It has been reported that the CaV3.1 T-type calcium channel may play an important role in trigeminal pain and a recent study identified a new missense mutation in the CACNA1G gene that encodes the pore forming α1 subunit of the CaV3.1 calcium channel. The mutation leads to a substitution of an Arginine (R) by a Glutamine (Q) at position 706 in the I-II linker region of the channel. Here, we used whole-cell voltage-clamp recordings to evaluate the biophysical properties of CaV3.1 wild-type and R706Q mutant channels expressed in tsA-201 cells. Our data indicate an increase in current density in the R706Q mutant, leading to a gain-of-function effect, without changes in the voltage for half activation. Moreover, voltage clamp using an action potential waveform protocol revealed an increase in the tail current at the repolarization phase in the R706Q mutant. No changes were observed in the voltage-dependence of inactivation. However, the R706Q mutant displayed a faster recovery from inactivation. Hence, the gain-of-function effects in the R706Q CaV3.1 mutant have the propensity to impact pain transmission in the trigeminal system, consistent with a contribution to trigeminal neuralgia pathophysiology.




Similar content being viewed by others
Data availability
The data used in our study are available from the authors on reasonable request.
Abbreviations
- TN:
-
Trigeminal neuralgia
- WT:
-
Wild type
- VGCCs:
-
Voltage-gated calcium channels
References
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR (2010) A method and server for predicting damaging missense mutations. Nat Methods 7:248–249. https://doi.org/10.1038/nmeth0410-248
Arias O II, Vitko I, Fortuna M, Baumgart JP, Sokolova S, Shumilin IA, Van Deusen A, Soriano-Garcia M, Gomora JC, Perez-Reyes E (2008) Characterization of the gating brake in the I-II loop of Ca(v)3.2 T-type Ca(2+) channels. J Biol Chem 283:8136–8144. https://doi.org/10.1074/jbc.M708761200
Baumgart JP, Vitko I, Bidaud I, Kondratskyi A, Lory P, Perez-Reyes E (2008) I-II loop structural determinants in the gating and surface expression of low voltage-activated calcium channels. PLoS One 3:e2976. https://doi.org/10.1371/journal.pone.0002976
Bendtsen L, Zakrzewska JM, Heinskou TB, Hodaie M, Leal PRL, Nurmikko T, Obermann M, Cruccu G, Maarbjerg S (2020) Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol 19:784–796. https://doi.org/10.1016/S1474-4422(20)30233-7
Cain SM, Snutch TP (2010) Contributions of T-type calcium channel isoforms to neuronal firing. Channels (Austin) 4:475–482. https://doi.org/10.4161/chan.4.6.14106
Chemin J, Monteil A, Bourinet E, Nargeot J, Lory P (2001) Alternatively spliced alpha(1G) (Ca(V)3.1) intracellular loops promote specific T-type Ca(2+) channel gating properties. Biophys J 80:1238–1250. https://doi.org/10.1016/S0006-3495(01)76100-0
Chemin J, Siquier-Pernet K, Nicouleau M, Barcia G, Ahmad A, Medina-Cano D, Hanein S, Altin N, Hubert L, Bole-Feysot C, Fourage C, Nitschke P, Thevenon J, Rio M, Blanc P, Vidal C, Bahi-Buisson N, Desguerre I, Munnich A et al (2018) De novo mutation screening in childhood-onset cerebellar atrophy identifies gain-of-function mutations in the CACNA1G calcium channel gene. Brain 141:1998–2013. https://doi.org/10.1093/brain/awy145
Choi S, Yu E, Hwang E, Llinas RR (2016) Pathophysiological implication of CaV3.1 T-type Ca2+ channels in trigeminal neuropathic pain. Proc Natl Acad Sci U S A 113:2270–2275. https://doi.org/10.1073/pnas.1600418113
Cruccu G, Finnerup NB, Jensen TS, Scholz J, Sindou M, Svensson P, Treede RD, Zakrzewska JM, Nurmikko T (2016) Trigeminal neuralgia: new classification and diagnostic grading for practice and research. Neurology 87:220–228. https://doi.org/10.1212/WNL.0000000000002840
Devor M, Amir R, Rappaport ZH (2002) Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain 18:4–13. https://doi.org/10.1097/00002508-200201000-00002
Di Stefano G, Yuan JH, Cruccu G, Waxman SG, Dib-Hajj SD, Truini A (2020) Familial trigeminal neuralgia—a systematic clinical study with a genomic screen of the neuronal electrogenisome. Cephalalgia 40:767–777. https://doi.org/10.1177/0333102419897623
Dong W, Jin SC, Allocco A, Zeng X, Sheth AH, Panchagnula S, Castonguay A, Lorenzo LE, Islam B, Brindle G, Bachand K, Hu J, Sularz A, Gaillard J, Choi J, Dunbar A, Nelson-Williams C, Kiziltug E, Furey CG et al (2020) Exome sequencing implicates impaired GABA signaling and neuronal ion transport in trigeminal neuralgia. iScience 23:101552. https://doi.org/10.1016/j.isci.2020.101552
Eide PK (2022) Familial occurrence of classical and idiopathic trigeminal neuralgia. J Neurol Sci 434:120101. https://doi.org/10.1016/j.jns.2021.120101
Gambeta E, Chichorro JG, Zamponi GW (2020) Trigeminal neuralgia: an overview from pathophysiology to pharmacological treatments. Mol Pain 16:1744806920901890. https://doi.org/10.1177/1744806920901890
Gambeta E, Gandini MA, Souza IA, Ferron L, Zamponi GW (2021) A CACNA1A variant associated with trigeminal neuralgia alters the gating of Cav2.1 channels. Mol. Brain 14:4. https://doi.org/10.1186/s13041-020-00725-y
Gambeta E, Gandini MA, Souza IA, Zamponi GW (2022) Ca V 3.2 calcium channels contribute to trigeminal neuralgia. Pain 163:2315–2325. https://doi.org/10.1097/j.pain.0000000000002651
Harding EK, Zamponi GW (2022) Central and peripheral contributions of T-type calcium channels in pain. Mol Brain 15:39. https://doi.org/10.1186/s13041-022-00923-w
Iftinca MC, Zamponi GW (2009) Regulation of neuronal T-type calcium channels. Trends Pharmacol Sci 30:32–40. https://doi.org/10.1016/j.tips.2008.10.004
Maarbjerg S, Benoliel R (2021) The changing face of trigeminal neuralgia—a narrative review. Headache 61:817–837. https://doi.org/10.1111/head.14144
Marchenkova A, van den Maagdenberg AM, Nistri A (2016) Loss of inhibition by brain natriuretic peptide over P2X3 receptors contributes to enhanced spike firing of trigeminal ganglion neurons in a mouse model of familial hemiplegic migraine type-1. Neuroscience 331:197–205. https://doi.org/10.1016/j.neuroscience.2016.06.034
McCobb DP, Beam KG (1991) Action potential waveform voltage-clamp commands reveal striking differences in calcium entry via low and high voltage-activated calcium channels. Neuron 7:119–127. https://doi.org/10.1016/0896-6273(91)90080-j
Morino H, Matsuda Y, Muguruma K, Miyamoto R, Ohsawa R, Ohtake T, Otobe R, Watanabe M, Maruyama H, Hashimoto K, Kawakami H (2015) A mutation in the low voltage-gated calcium channel CACNA1G alters the physiological properties of the channel, causing spinocerebellar ataxia. Mol Brain 8:89. https://doi.org/10.1186/s13041-015-0180-4
Perez-Reyes E (2003) Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev 83:117–161. https://doi.org/10.1152/physrev.00018.2002
Puil E, Miura RM, Spigelman I (1989) Consequences of 4-aminopyridine applications to trigeminal root ganglion neurons. J Neurophysiol 62:810–820. https://doi.org/10.1152/jn.1989.62.3.810
Rappaport ZH, Devor M (1994) Trigeminal neuralgia: the role of self-sustaining discharge in the trigeminal ganglion. Pain 56:127–138. https://doi.org/10.1016/0304-3959(94)90086-8
Shcheglovitov A, Vitko I, Bidaud I, Baumgart JP, Navarro-Gonzalez MF, Grayson TH, Lory P, Hill CE, Perez-Reyes E (2008) Alternative splicing within the I-II loop controls surface expression of T-type Ca(v)3.1 calcium channels. FEBS Lett 582:3765–3770. https://doi.org/10.1016/j.febslet.2008.10.013
Simms BA, Zamponi GW (2014) Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron 82:24–45. https://doi.org/10.1016/j.neuron.2014.03.016
Tseng WT, Tsai ML, Iwata K, Yen CT (2012) Long-term changes in trigeminal ganglionic and thalamic neuronal activities following inferior alveolar nerve transection in behaving rats. J Neurosci 32:16051–16063. https://doi.org/10.1523/JNEUROSCI.1828-12.2012
Vitko I, Bidaud I, Arias JM, Mezghrani A, Lory P, Perez-Reyes E (2007) The I-II loop controls plasma membrane expression and gating of Ca(v)3.2 T-type Ca2+ channels: a paradigm for childhood absence epilepsy mutations. J Neurosci 27:322–330. https://doi.org/10.1523/JNEUROSCI.1817-06.2007
Weiss N, Black SA, Bladen C, Chen L, Zamponi GW (2013) Surface expression and function of Cav3.2 T-type calcium channels are controlled by asparagine-linked glycosylation. Pflugers Arch 465:1159–1170. https://doi.org/10.1007/s00424-013-1259-3
Weiss N, Hameed S, Fernandez-Fernandez JM, Fablet K, Karmazinova M, Poillot C, Proft J, Chen L, Bidaud I, Monteil A, Huc-Brandt S, Lacinova L, Lory P, Zamponi GW, De Waard M (2012) A Ca(v)3.2/syntaxin-1A signaling complex controls T-type channel activity and low-threshold exocytosis. J Biol Chem 287:2810–2818. https://doi.org/10.1074/jbc.M111.290882
Weiss N, Zamponi GW (2020) Genetic T-type calcium channelopathies. J Med Genet 57:1–10. https://doi.org/10.1136/jmedgenet-2019-106163
Acknowledgements
We are grateful to Lina Chen for her technical support.
Code availability
Not applicable.
Funding
This work was supported by a Project Grant from the Canadian Institutes of Health Research (CIHR) to GWZ. EG held a Alberta Innovates Scholarship. GWZ is a Canada Research Chair.
Author information
Authors and Affiliations
Contributions
AMA and EG performed experiments and data analysis. EG designed the study and drafted the manuscript. GWZ supervised the study and co-wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alaklabi, A.M., Gambeta, E. & Zamponi, G.W. Electrophysiological characterization of a CaV3.1 calcium channel mutation linked to trigeminal neuralgia. Pflugers Arch - Eur J Physiol 475, 711–718 (2023). https://doi.org/10.1007/s00424-023-02808-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-023-02808-w