Abstract
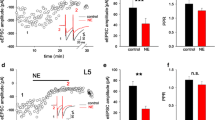
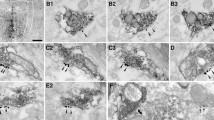
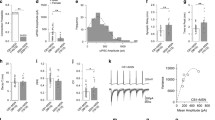
The nucleus accumbens (NAc) receives cortical projections principally from the insular cortex (IC) and medial prefrontal cortex (mPFC). Among NAc neurons, cholinergic interneurons (ChNs) regulate the activities of medium spiny neurons (MSNs), which make up ~ 95% of NAc neurons, by modulating their firing and synaptic properties. However, little is known about the synaptic mechanisms, including their cell-type-dependent corticoaccumbal projection properties and cholinergic effects on the NAc core. Here, we performed whole-cell patch-clamp recordings from NAc MSNs and ChNs in acute brain slice preparations obtained from rats that received an AAV5-hSyn-ChR2(H134R)-mCherry injection into the IC or mPFC. Light stimulation of IC or mPFC axons induced comparable phase-locked excitatory postsynaptic currents (EPSCs) in MSNs. On the other hand, ChNs showed consistent EPSCs evoked by light stimulation of mPFC axons, whereas light stimulation of IC axons evoked much smaller EPSCs, which often showed failure in ChNs. Light-evoked EPSCs were abolished by tetrodotoxin and were recovered by 4-aminopyridine, suggesting that corticoaccumbal projections monosynaptically induce EPSCs in MSNs and ChNs. Carbachol effectively suppressed the amplitude of EPSCs in MSNs and ChNs evoked by light stimulation of IC or mPFC axons and in ChNs evoked by stimulating mPFC axons. The carbachol-induced suppression was recovered by atropine or pirenzepine, while preapplication of gallamine, J104129, PD102807, or AF-DX384 did not block the carbachol-induced EPSC suppression. These results suggest that NAc MSNs and ChNs are differentially regulated by excitatory projections from the IC and mPFC and that these corticoaccumbal excitatory inputs are modulated by M1 receptor activation.







Similar content being viewed by others
Abbreviations
- AAV:
-
Adeno-associated virus
- ACSF:
-
Artificial cerebrospinal fluid
- ChNs:
-
Cholinergic interneurons
- ChR2:
-
Channelrhodopsin-2
- DNQX:
-
6,7-Dinitroquinoxaline-2,3-dione
- EPSCs:
-
Excitatory postsynaptic currents
- FSNs:
-
Fast-spiking neurons
- IC:
-
Insular cortex
- mPFC:
-
Medial prefrontal cortex
- MSNs:
-
Medium spiny neurons
- NAc:
-
Nucleus accumbens
- TTX:
-
Tetrodotoxin
- VGAT:
-
Vesicular GABA transporter
- 4-AP:
-
4-Aminopyridine
References
Arguello AA, Wang R, Lyons CM, Higginbotham JA, Hodges MA, Fuchs RA (2017) Role of the agranular insular cortex in contextual control over cocaine-seeking behavior in rats. Psychopharmacology 234:2431–2441
Brog JS, Salyapongse A, Deutch AY, Zahm DS (1993) The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338:255–278
de Rover M, Lodder JC, Kits KS, Schoffelmeer AN, Brussaard AB (2002) Cholinergic modulation of nucleus accumbens medium spiny neurons. Eur J Neurosci 16:2279–2290
Di Chiara G (2002) Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res 137:75–114
Ebihara K, Yamamoto K, Ueda K, Koshikawa N, Kobayashi M (2013) ChNs suppress action potential initiation of medium spiny neurons in rat nucleus accumbens shell. Neuroscience 236:332–344
Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM (2013) BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79:658–664
Groenewegen HJ, Room P, Witter MP, Lohman AH (1982) Cortical afferents of the nucleus accumbens in the cat, studied with anterograde and retrograde transport techniques. Neuroscience 7:977–996
Horinuki E, Shinoda M, Shimizu N, Koshikawa N, Kobayashi M (2015) Orthodontic force facilitates cortical responses to periodontal stimulation. J Dent Res 94:1158–1166
Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT (2003) Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature 424:316–320
Kawaguchi Y, Otsuka T, Morishima M, Ushimaru M, Kubota Y (2019) Control of excitatory hierarchical circuits by parvalbumin-FS basket cells in layer 5 of the frontal cortex: insights for cortical oscillations. J Neurophysiol 121:2222–2236
Kobayashi M, Cui Y, Sako T, Sasabe T, Mizoguchi N, Yamamoto K, Wada Y, Kataoka Y, Koshikawa N (2013) Functional neuroimaging of aversive taste-related areas in the alert rat revealed by positron emission tomography. J Neurosci Res 91:1363–1370
Kohnomi S, Koshikawa N, Kobayashi M (2012) D2-like dopamine receptors differentially regulate unitary IPSCs depending on presynaptic GABAergic neuron subtypes in rat nucleus accumbens shell. J Neurophysiol 107:692–703
Kubista H, Kosenburger K, Mahlknecht P, Drobny H, Boehm S (2009) Inhibition of transmitter release from rat sympathetic neurons via presynaptic M1 muscarinic acetylcholine receptors. Br J Pharmacol 156:1342–1352
Mark GP, Weinberg JB, Rada PV, Hoebel BG (1995) Extracellular acetylcholine is increased in the nucleus accumbens following the presentation of an aversively conditioned taste stimulus. Brain Res 688:184–188
Mateo Y, Johnson KA, Covey DP, Atwood BK, Wang HL, Zhang S, Gildish I, Cachope R, Bellocchio L, Guzman M, Morales M, Cheer JF, Lovinger DM (2017) Endocannabinoid actions on cortical terminals orchestrate local modulation of dopamine release in the nucleus accumbens. Neuron 96:1112–1126
Murayama S, Yamamoto K, Fujita S, Takei H, Inui T, Ogiso B, Kobayashi M (2019) Extracellular glucose-dependent IPSC enhancement by leptin in fast-spiking to pyramidal neuron connections via JAK2-PI3K pathway in the rat insular cortex. Neuropharmacology 149:133–148
Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A (2002) A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20:87–90
Nakamura H, Kato R, Shirakawa T, Koshikawa N, Kobayashi M (2015) Spatiotemporal profiles of dental pulp nociception in rat cerebral cortex: an optical imaging study. J Comp Neurol 523:1162–1174
Phillipson OT, Griffiths AC (1985) The topographic order of inputs to nucleus accumbens in the rat. Neuroscience 16:275–296
Pushparaj A, Hamani C, Yu W, Shin DS, Kang B, Nobrega JN, Le Foll B (2013) Electrical stimulation of the insular region attenuates nicotine-taking and nicotine-seeking behaviors. Neuropsychopharmacology 38:690–698
Ramírez-Lugo L, Núñez-Jaramillo L, Bermúdez-Rattoni F (2007) Taste memory formation: role of nucleus accumbens. Chem Senses 32:93–97
Rogers-Carter MM, Djerdjaj A, Gribbons KB, Varela JA, Christianson JP (2019) Insular cortex projections to nucleus accumbens core mediate social approach to stressed juvenile rats. J Neurosci 39:8717–8729
Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW (2013) Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci 16:1094–1100
Sesack SR, Deutch AY, Roth RH, Bunney BS (1989) Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 290:213–242
Taverna S, Canciani B, Pennartz CM (2007) Membrane properties and synaptic connectivity of fast-spiking interneurons in rat ventral striatum. Brain Res 1152:49–56
Uematsu M, Hirai Y, Karube F, Ebihara S, Kato M, Abe K, Obata K, Yoshida S, Hirabayashi M, Yanagawa Y, Kawaguchi Y (2008) Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb Cortex 18:315–330
Usui M, Kaneko K, Oi Y, Kobayashi M (2019) Orexin facilitates GABAergic IPSCs via postsynaptic OX1 receptors coupling to the intracellular PKC signalling cascade in the rat cerebral cortex. Neuropharmacology 149:97–112
Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, Morales M, Epstein DH, Shaham Y (2018) Volitional social interaction prevents drug addiction in rat models. Nat Neurosci 21:1520–1529
Weikop P, Jensen KL, Thomsen M (2020) Effects of muscarinic M1 receptor stimulation on reinforcing and neurochemical effects of cocaine in rats. Neuropsychopharmacology 45:1994–2002
Wess J, Lambrecht G, Mutschler E, Brann MR, Dörje F (1991) Selectivity profile of the novel muscarinic antagonist UH-AH 37 determined by the use of cloned receptors and isolated tissue preparations. Br J Pharmacol 102:246–250
Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K (2010) ChNs control local circuit activity and cocaine conditioning. Science 330:1677–1681
Yamamoto T (1984) Taste responses of cortical neurons. Prog Neurobiol 23:273–315
Yamamoto K, Ebihara K, Koshikawa N, Kobayashi M (2013) Reciprocal regulation of inhibitory synaptic transmission by nicotinic and muscarinic receptors in rat nucleus accumbens shell. J Physiol 591:5745–5763
Yamamoto K, Kobayashi M (2018) Opposite roles in short-term plasticity for N-type and P/Q-type voltage-dependent calcium channels in GABAergic neuronal connections in the rat cerebral cortex. J Neurosci 38:9814–9828
Yasui Y, Breder CD, Saper CB, Cechetto DF (1991) Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol 303:355–374
Zhang Y, Kaneko R, Yanagawa Y, Saito Y (2014) The vestibulo- and preposito-cerebellar cholinergic neurons of a ChAT-tdTomato transgenic rat exhibit heterogeneous firing properties and the expression of various neurotransmitter receptors. Eur J Neurosci 39:1294–1313
Zhang L, Warren RA (2002) Muscarinic and nicotinic presynaptic modulation of EPSCs in the nucleus accumbens during postnatal development. J Neurophysiol 88:3315–3330
Zhou FM, Wilson C, Dani JA (2003) Muscarinic and nicotinic cholinergic mechanisms in the mesostriatal dopamine systems. Neuroscientist 9:23–36
Acknowledgements
VGAT-Venus transgenic rats were generated by Drs. Y. Yanagawa, M. Hirabayashi, and Y. Kawaguchi at the National Institute for Physiological Sciences, Okazaki, Japan, using pCS2-Venus provided by Dr. A. Miyawaki. We thank Prof. S. Fujita for the statistical analysis.
Funding
This work was supported by KAKENHI grant nos. 18K17019 and 21K16938 to Y.N., grant no. 17K11653 to K.Y., and grant no. 19H03821 to M.K. from the MEXT, Japan; grants from the Sato and Uemura foundations and Dental Research Center at Nihon University School of Dentistry also supported this work.
Author information
Authors and Affiliations
Contributions
Yuka Nakaya, Kiyofumi Yamamoto, and Masayuki Kobayashi conceived and designed the research. Kensuke Hirose, Yuka Nakaya, Kohei Kitano, and Kiyofumi Yamamoto conducted experiments. Yasuhiko Saito, Ryosuke Kaneko, and Yuchio Yanagawa developed the choline acetyltransferase (ChAT)-tdTomato transgenic rat. Data analyses were performed by Kensuke Hirose, Yuka Nakaya, Kohei Kitano, Kiyofumi Yamamoto, and Masayuki Kobayashi. The draft of the manuscript was written by Kensuke Hirose, Yuka Nakaya, and Kiyofumi Yamamoto, Tetsuo Shirakawa, and Masayuki Kobayashi. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution where the experiments were performed. All experiments were approved by the Animal Experimentation Committee at Nihon University and were carried out according to the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hirose, K., Nakaya, Y., Kitano, K. et al. Differential regulation of medium spiny and cholinergic neurons in the nucleus accumbens core by the insular and medial prefrontal cortices in the rat. Pflugers Arch - Eur J Physiol 473, 1911–1924 (2021). https://doi.org/10.1007/s00424-021-02634-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-021-02634-y