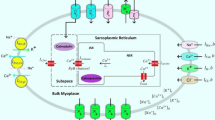
Abstract
Excitation-contraction coupling from the integration of action potential duration (APD) and muscle contractility plays an important role in arrhythmogenesis. We aimed to determine whether distinctive excitation-contraction coupling contributes to the genesis of ventricular tachycardias (VTs). Action potential (AP) and mechanical activity were simultaneously recorded under electrical pacing (cycle lengths from 1000 to 100 ms) in the tissue model created from isolated rabbit right ventricular outflow tracts treated with NS 5806 (10 μM, transient outward potassium current enhancer), pinacidil (2 μM, ATP-sensitive potassium channel opener), and pilsicainide (5 μM, sodium channel blocker). There were 15 (9.9%) inducible VT episodes (group 1) and 136 (90.1%) non-inducible VT episodes (group 2) in our tissue model. Group 1 had greater post-pacing increases of the first occurrence of AP at 90% repolarization (ΔAPD90, p < 0.001) and contractility (ΔContractility, p = 0.003) compared with group 2. Triggered VT episodes were common (72.7%) in cases with a ΔAPD90 > 15% and a ΔContractility > 270%, but were undetectable in those with a ΔAPD90 < 15% and a ΔContractility < 270%. In those with pacing-induced VTs, KB-R7943 (10 μM, a Na+–Ca2+ exchanger inhibitor, NCX inhibitor) significantly reduced the occurrence of VTs from 100.0 to 20.0% (15/15 to 3/15 episodes, p < 0.001). Concurrent increases in both post-pacing APD and contractility resulted in the occurrence of ventricular arrhythmias. NCX inhibition may be a potential therapeutic strategy for ventricular arrhythmias.





Similar content being viewed by others
Abbreviations
- AP:
-
action potential
- APA:
-
action potential amplitude
- APD:
-
action potential duration
- DAD:
-
delayed afterdepolarization
- EAD:
-
early afterdepolarization
- E-C:
-
excitation-contraction
- M-E:
-
mechano-electric
- NCX:
-
Na+–Ca2+ exchanger
- NIH:
-
National Institutes of Health
- RV:
-
right ventricle
- RVOT:
-
right ventricular outflow tract
- RyR:
-
ryanodine receptor
- SEM:
-
standard error of the mean
- SPSS:
-
Statistical Package for the Social Sciences
- SR:
-
sarcoplasm reticulum
- VA:
-
ventricular arrhythmia
- VT:
-
ventricular tachycardia
- ΔAPD90 :
-
first occurrence of post-pacing APD90 prolongation
- ΔContractility:
-
first occurrence of post-pacing contractility enhancement
References
Asakura K, Cha CY, Yamaoka H, Horikawa Y, Memida H, Powell T, Amano A, Noma A (2014) EAD and DAD mechanisms analyzed by developing a new human ventricular cell model. Prog Biophys Mol Biol 116:11–24. https://doi.org/10.1016/j.pbiomolbio.2014.08.008
Bjerregaard P, Gussak I (2013) Short QT Syndrome. In: Gussak I, Antzelevitch C (eds) Electrical diseases of the heart: volume 1: basic foundations and primary electrical diseases. Springer London, London, pp 569–581. https://doi.org/10.1007/978-1-4471-4881-4_33
Brugada J, Campuzano O, Arbelo E, Sarquella-Brugada G, Brugada R (2018) Present status of Brugada syndrome: JACC state-of-the-art review. J Am Coll Cardiol 72:1046–1059. https://doi.org/10.1016/j.jacc.2018.06.037
Calloe K, Cordeiro JM, Di Diego JM, Hansen RS, Grunnet M, Olesen SP, Antzelevitch C (2009) A transient outward potassium current activator recapitulates the electrocardiographic manifestations of Brugada syndrome. Cardiovasc Res 81:686–694. https://doi.org/10.1093/cvr/cvn339
Chang SL, Chen YC, Chen YJ, Wangcharoen W, Lee SH, Lin CI, Chen SA (2007) Mechanoelectrical feedback regulates the arrhythmogenic activity of pulmonary veins. Heart 93:82–88. https://doi.org/10.1136/hrt.2006.089359
Council NR (2011) Guide for the care and use of laboratory animals, 8th edn. The National Academies Press, Washington, DC. https://doi.org/10.17226/12910
Eisner DA, Caldwell JL, Kistamas K, Trafford AW (2017) Calcium and excitation-contraction coupling in the heart. Circ Res 121:181–195. https://doi.org/10.1161/circresaha.117.310230
Fu M, Wu M, Wang J-F, Qiao Y-J, Wang Z (2007) Disruption of the intracellular Ca2+ homeostasis in the cardiac excitation-contraction coupling is a crucial mechanism of arrhythmic toxicity in aconitine-induced cardiomyocytes. Biochem Biophys Res Commun 354:929–936
Gorski PA, Kho C, Oh JG (2018) Measuring cardiomyocyte contractility and calcium handling in vitro. Methods Mol Biol (Clifton, NJ) 1816:93–104. https://doi.org/10.1007/978-1-4939-8597-5_7
Jafri MS (2012) Models of excitation-contraction coupling in cardiac ventricular myocytes. Methods Mol Biol (Clifton, NJ) 910:309–335. https://doi.org/10.1007/978-1-61779-965-5_14
Kannankeril P, Roden DM, Darbar D (2010) Drug-induced long QT syndrome. Pharmacol Rev 62:760–781. https://doi.org/10.1124/pr.110.003723
Kohl P, Sachs F, Franz MR (2011) Cardiac mechano-electric coupling and arrhythmias. OUP, Oxford
Lu YY, Chung FP, Chen YC, Tsai CF, Kao YH, Chao TF, Huang JH, Chen SA, Chen YJ (2014) Distinctive electrophysiological characteristics of right ventricular out-flow tract cardiomyocytes. J Cell Mol Med 18:1540–1548. https://doi.org/10.1111/jcmm.12329
Luo CH, Rudy Y (1994) A dynamic model of the cardiac ventricular action potential. II. Afterdepolarizations, triggered activity, and potentiation. Circ Res 74:1097–1113. https://doi.org/10.1161/01.res.74.6.1097
Monasky MM, Pappone C, Piccoli M, Ghiroldi A, Micaglio E, Anastasia L (2018) Calcium in Brugada Syndrome: questions for future research. Front Physiol 9:1088. https://doi.org/10.3389/fphys.2018.01088
Morita H, Morita ST, Nagase S, Banba K, Nishii N, Tani Y, Watanabe A, Nakamura K, Kusano KF, Emori T, Matsubara H, Hina K, Kita T, Ohe T (2003) Ventricular arrhythmia induced by sodium channel blocker in patients with Brugada syndrome. J Am Coll Cardiol 42:1624–1631. https://doi.org/10.1016/j.jacc.2003.06.004
Nademanee K, Veerakul G, Chandanamattha P, Chaothawee L, Ariyachaipanich A, Jirasirirojanakorn K, Likittanasombat K, Bhuripanyo K, Ngarmukos T (2011) Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation 123:1270–1279. https://doi.org/10.1161/circulationaha.110.972612
Schober T, Huke S, Venkataraman R, Gryshchenko O, Kryshtal D, Hwang HS, Baudenbacher FJ, Knollmann BC (2012) Myofilament Ca sensitization increases cytosolic Ca binding affinity, alters intracellular Ca homeostasis, and causes pause-dependent Ca-triggered arrhythmia. Circ Res 111:170–179. https://doi.org/10.1161/circresaha.112.270041
Shimizu W, Antzelevitch C (2000) Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol 35:778–786. https://doi.org/10.1016/s0735-1097(99)00582-3
Sipido KR (2004) Understanding cardiac alternans: the answer lies in the Ca2+ store. Circ Res 94:570–572. https://doi.org/10.1161/01.res.0000124606.14903.6f
Sipido KR, Bito V, Antoons G, Volders PG, Vos MA (2007) Na/Ca exchange and cardiac ventricular arrhythmias. Ann N Y Acad Sci 1099:339–348. https://doi.org/10.1196/annals.1387.066
Tang L, Joung B, Ogawa M, Chen PS, Lin SF (2012) Intracellular calcium dynamics, shortened action potential duration, and late-phase 3 early afterdepolarization in Langendorff-perfused rabbit ventricles. J Cardiovasc Electrophysiol 23:1364–1371. https://doi.org/10.1111/j.1540-8167.2012.02400.x
Tsai WC, Lu YY, Chen YC, Chang CJ, Kao YH, Lin YK, Chen YH, Chen SA, Yang LY, Chen YJ (2015) Ablation of androgen receptor gene triggers right ventricular outflow tract ventricular tachycardia. Int J Cardiol 189:172–181. https://doi.org/10.1016/j.ijcard.2015.04.080
Tse G, Chan YW, Keung W, Yan BP (2017) Electrophysiological mechanisms of long and short QT syndromes. Int J Cardiol Heart Vasc 14:8–13. https://doi.org/10.1016/j.ijcha.2016.11.006
Venetucci LA, Trafford AW, O’Neill SC, Eisner DA (2008) The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc Res 77:285–292. https://doi.org/10.1093/cvr/cvm009
Wongcharoen W, Chen YC, Chen YJ, Chang CM, Yeh HI, Lin CI, Chen SA (2006) Effects of a Na+/Ca2+ exchanger inhibitor on pulmonary vein electrical activity and ouabain-induced arrhythmogenicity. Cardiovasc Res 70:497–508. https://doi.org/10.1016/j.cardiores.2006.02.026
Zeng J, Rudy Y (1995) Early afterdepolarizations in cardiac myocytes: mechanism and rate dependence. Biophys J 68:949–964. https://doi.org/10.1016/s0006-3495(95)80271-7
Zima AV, Bovo E, Mazurek SR, Rochira JA, Li W, Terentyev D (2014) Ca handling during excitation-contraction coupling in heart failure. Pflugers Arch 466:1129–1137. https://doi.org/10.1007/s00424-014-1469-3
Funding
This work was supported by grants from the Ministry of Science and Technology (MOST105-2314-B-016-035-MY3, MOST105-2628-B-038-012-MY3, MOST105-2314-B-038-059-MY3, 107-2314-B-281-009, 107-2314-B-038-101-MY3), Taipei Medical University-Wan Fang Hospital (106-eva-02, 106-eva-06, 106-swf-01, 107-wf-swf-01, and 107-wf-eva-01), Cathay General Hospital (107CGH-TMU-05), Chi-Mei Medical Center (107CM-TMU-04), and the Foundation for the Development of Internal Medicine in Okinawa (31-02-003).
Author information
Authors and Affiliations
Contributions
CML and YJC generated the research topic, did the literature search, and prepared the first manuscript. CML and FZL did the electropharmacological experiments and ran the statistical tests. CML, FZL, YCC, and YJC analyzed and interpreted the data. YKL, YYL, CIW, SH, and SAC conceived the study concept and contributed important parts in data interpretation. YJC critically read the manuscript and made vital suggestions in revision. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The investigation was approved by the local ethics review board of Taipei Veterans General Hospital (reference number: IACUC-2019-192).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, CM., Lin, FZ., Chen, YC. et al. Concurrent increases in post-pacing action potential duration and contractility predict occurrence of ventricular arrhythmia. Pflugers Arch - Eur J Physiol 472, 1783–1791 (2020). https://doi.org/10.1007/s00424-020-02445-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02445-7