Abstract
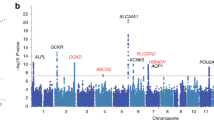
The nature and importance of genetic factors regulating the differential handling of Ca2+ and Mg2+ by the renal tubule in the general population are poorly defined. We conducted a genome-wide meta-analysis of urinary magnesium-to-calcium ratio to identify associated common genetic variants. We included 9320 adults of European descent from four genetic isolates and three urban cohorts. Urinary magnesium and calcium concentrations were measured centrally in spot urine, and each study conducted linear regression analysis of urinary magnesium-to-calcium ratio on ~2.5 million single-nucleotide polymorphisms (SNPs) using an additive model. We investigated, in mouse, the renal expression profile of the top candidate gene and its variation upon changes in dietary magnesium. The genome-wide analysis evidenced a top locus (rs172639, p = 1.7 × 10−12), encompassing CLDN14, the gene coding for claudin-14, that was genome-wide significant when using urinary magnesium-to-calcium ratio, but not either one taken separately. In mouse, claudin-14 is expressed in the distal nephron segments specifically handling magnesium, and its expression is regulated by chronic changes in dietary magnesium content. A genome-wide approach identified common variants in the CLDN14 gene exerting a robust influence on the differential excretion of Mg2+ over Ca2+ in urine. These data highlight the power of urinary electrolyte ratios to unravel genetic determinants of renal tubular function. Coupled with mouse experiments, these results support a major role for claudin-14, a gene associated with kidney stones, in the differential paracellular handling of divalent cations by the renal tubule.




Similar content being viewed by others
References
Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER, Rasmussen J, Anderson JM, Dolan DF, Forge A, Raphael Y, Camper SA, Friedman TB (2003) Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet 12:2049–2061. doi:10.1093/hmg/ddg210
Blanchard A, Jeunemaitre X, Coudol P, Dechaux M, Froissart M, May A, Demontis R, Fournier A, Paillard M, Houillier P (2001) Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int 59:2206–2215. doi:10.1046/j.1523-1755.2001.0590062206.x
Bleich M, Shan Q, Himmerkus N (2012) Calcium regulation of tight junction permeability. Ann N Y Acad Sci 1258:93–99. doi:10.1111/j.1749-6632.2012.06539.x
Bonny O, Rubin A, Huang C-L, Frawley WH, Pak CYC, Moe OW (2008) Mechanism of urinary calcium regulation by urinary magnesium and pH. J Am Soc Nephrol 19:1530–1537. doi:10.1681/ASN.2007091038
Bushinsky DA, Favus MJ, Langman CB, Coe FL (1986) Mechanism of chronic hypercalciuria with furosemide: increased calcium absorption. Am J Phys 251:F17–F24
De Baaij JHF, Hoenderop JGJ, Bindels RJM (2015) Magnesium in man: implications for health and disease. Physiol Rev 95:1–46. doi:10.1152/physrev.00012.2014
De Groot T, Bindels RJM, Hoenderop JGJ (2008) TRPV5: an ingeniously controlled calcium channel. Kidney Int 74:1241–1246. doi:10.1038/ki.2008.320
Deary IJ, Gow AJ, Taylor MD, Corley J, Brett C, Wilson V, Campbell H, Whalley LJ, Visscher PM, Porteous DJ, Starr JM (2007) The Lothian birth cohort 1936: a study to examine influences on cognitive ageing from age 11 to age 70 and beyond. BMC Geriatr 7:28. doi:10.1186/1471-2318-7-28
Deary IJ, Gow AJ, Pattie A, Starr JM (2012) Cohort profile: the lothian birth cohorts of 1921 and 1936. Int J Epidemiol 41:1576–1584. doi:10.1093/ije/dyr197
Devuyst O, Pirson Y (2007) Genetics of hypercalciuric stone forming diseases. Kidney Int 72:1065–1072. doi:10.1038/sj.ki.5002441
Devuyst O, Knoers NVAM, Remuzzi G, Schaefer F (2014) Rare inherited kidney diseases: challenges, opportunities, and perspectives. Lancet 383:1844–1859. doi:10.1016/S0140-6736(14)60659-0
Dimke H, Desai P, Borovac J, Lau A, Pan W, Alexander RT (2013) Activation of the Ca(2+)-sensing receptor increases renal claudin-14 expression and urinary Ca(2+) excretion. Am J Physiol Renal Physiol 304:F761–F769. doi:10.1152/ajprenal.00263.2012
Elkouby-Naor L, Abassi Z, Lagziel A, Gow A, Ben-Yosef T (2008) Double gene deletion reveals lack of cooperation between claudin 11 and claudin 14 tight junction proteins. Cell Tissue Res 333:427–438. doi:10.1007/s00441-008-0621-9
Firmann M, Mayor V, Vidal PM, Bochud M, Pécoud A, Hayoz D, Paccaud F, Preisig M, Song KS, Yuan X, Danoff TM, Stirnadel HA, Waterworth D, Mooser V, Waeber G, Vollenweider P (2008) The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord 8:6. doi:10.1186/1471-2261-8-6
Glaudemans B, Terryn S, Gölz N, Brunati M, Cattaneo A, Bachi A, Al-Qusairi L, Ziegler U, Staub O, Rampoldi L, Devuyst O (2014) A primary culture system of mouse thick ascending limb cells with preserved function and uromodulin processing. Pflugers Arch 466:343–356. doi:10.1007/s00424-013-1321-1
Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J (2012) Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 31:1999–2012. doi:10.1038/emboj.2012.49
Groenestege WMT (2006) The epithelial Mg2+ channel transient receptor potential Melastatin 6 is regulated by dietary Mg2+ content and estrogens. J Am Soc Nephrol 17:1035–1043. doi:10.1681/ASN.2005070700
Himmerkus N, Shan Q, Goerke B, Hou J, Goodenough DA, Bleich M (2008) Salt and acid-base metabolism in claudin-16 knockdown mice: impact for the pathophysiology of FHHNC patients. Am J Physiol Renal Physiol 295:F1641–F1647. doi:10.1152/ajprenal.90388.2008
Hou J, Paul DL, Goodenough DA (2005) Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci 118:5109–5118. doi:10.1242/jcs.02631
Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, Bleich M, Goodenough DA (2007) Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem. doi:10.1074/jbc.M700632200
Hou J, Renigunta A, Konrad M, Gomes A, Schneeberger E, Paul D, Goodenough DA (2008) Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 118:619–628. doi:10.1172/JCI33970DS1
Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA (2009) Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A 106:15350–15355. doi:10.1073/pnas.0907724106
Houillier P (2014) Mechanisms and regulation of renal magnesium transport. Annu Rev Physiol 76:411–430. doi:10.1146/annurev-physiol-021113-170336
Ikari A, Hirai N, Shiroma M, Harada H, Sakai H, Hayashi H, Suzuki Y, Degawa M, Takagi K (2004) Association of paracellin-1 with ZO-1 augments the reabsorption of divalent cations in renal epithelial cells. J Biol Chem 279:54826–54832. doi:10.1074/jbc.M406331200
Jaya Kausalya P, Amasheh S, Günzel D, Wurps H, Müller D, Fromm M, Hunziker W (2006) Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of claudin-16. J Clin Invest 116:878–891. doi:10.1172/JCI26323
Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SCF, Nurnberg P, Weber S (2006) Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 79:949–957. doi:10.1086/508617
Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP (2006) Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38:1124–1132. doi:10.1038/ng1877
Meyer TE, Verwoert GC, Hwang SJ, Glazer NL, Smith AV, van Rooij FJA, Ehret GB, Boerwinkle E, Felix JF, Leak TS, Harris TB, Yang Q, Dehghan A, Aspelund T, Katz R, Homuth G, Kocher T, Rettig R, Ried JS, Gieger C, Prucha H, Pfeufer A, Meitinger T, Coresh J, Hofman A, Sarnak MJ, Chen YDI, Uitterlinden AG, Chakravarti A, Psaty BM, van Duijn CM, Linda-Kao WH, Witteman JCM, Gudnason V, Siscovick DS, Fox CS, Köttgen A (2010) Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLoS Genet. doi:10.1371/journal.pgen.1001045
Müller D, Kausalya PJ, Claverie-Martin F, Meij IC, Eggert P, Garcia-Nieto V, Hunziker W (2003) A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am J Hum Genet 73:1293–1301. doi:10.1086/380418
Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M (2010) Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci U S A 107:8011–8016. doi:10.1073/pnas.0912901107
O’Seaghdha CM, Wu H, Yang Q, Kapur K, Guessous I, Zuber AM, Köttgen A, Stoudmann C, Teumer A, Kutalik Z, Mangino M, Dehghan A, Zhang W, Eiriksdottir G, Li G, Tanaka T, Portas L, Lopez LM, Hayward C, Lohman K, Matsuda K, Padmanabhan S, Firsov D, Sorice R, Ulivi S, Brockhaus AC, Kleber ME, Mahajan A, Ernst FD, Gudnason V, Launer LJ, Mace A, Boerwinckle E, Arking DE, Tanikawa C, Nakamura Y, Brown MJ, Gaspoz JM, Theler JM, Siscovick DS, Psaty BM, Bergmann S, Vollenweider P, Vitart V, Wright AF, Zemunik T, Boban M, Kolcic I, Navarro P, Brown EM, Estrada K, Ding J, Harris TB, Bandinelli S, Hernandez D, Singleton AB, Girotto G, Ruggiero D, d’Adamo AP, Robino A, Meitinger T, Meisinger C, Davies G, Starr JM, Chambers JC, Boehm BO, Winkelmann BR, Huang J, Murgia F, Wild SH, Campbell H, Morris AP, Franco OH, Hofman A, Uitterlinden AG, Rivadeneira F, Völker U, Hannemann A, Biffar R, Hoffmann W, Shin SY, Lescuyer P, Henry H, Schurmann C, Munroe PB, Gasparini P, Pirastu N, Ciullo M, Gieger C, März W, Lind L, Spector TD, Smith AV, Rudan I, Wilson JF, Polasek O, Deary IJ, Pirastu M, Ferrucci L, Liu Y, Kestenbaum B, Kooner JS, Witteman JCM, Nauck M, Kao WHL, Wallaschofski H, Bonny O, Fox CS, Bochud M (2013) Meta-analysis of genome-wide association studies identifies six new loci for serum calcium concentrations. PLoS Genet. doi:10.1371/journal.pgen.1003796
Polašek O, Marušić A, Rotim K, Hayward C, Vitart V, Huffman J, Campbell S, Janković S, Boban M, Biloglav Z, Kolčić I, Krželj V, Terzić J, Matec L, Tometić G, Nonković D, Ninčević J, Pehlić M, Žedelj J, Velagić V, Juričić D, Kirac I, Belak Kovačević S, Wright AF, Campbell H, Rudan I (2009) Genome-wide association study of anthropometric traits in Korčula Island, Croatia. Croat Med J 50:7–16. doi:10.3325/cmj.2009.50.7
Praga M, Vara J, González-Parra E, Andrés A, Alamo C, Araque A, Ortiz A, Rodicio JL (1995) Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Kidney Int 47:1419–1425. doi:10.1038/ki.1995.199
Quamme GA (1997) Renal magnesium handling: new insights in understanding old problems. Kidney Int 52:1180–1195. doi:10.1038/ki.1997.443
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Rudan I, Marušić A, Janković S, Rotim K, Boban M, Lauc G, Grković I, Đogaš Z, Zemunik T, Vatavuk Z, Benčić G, Rudan D, Mulić R, Krželj V, Terzić J, Stojanović D, Puntarić D, Bilić E, Ropac D, Vorko-Jović A, Znaor A, Stevanović R, Biloglav Z, Polašek O (2009) “10 001 Dalmatians:” Croatia launches its National Biobank. Croat Med J 50:4–6. doi:10.3325/cmj.2009.50.4
Schweigel-Röntgen M (2014) The families of zinc (SLC30 and SLC39) and copper (SLC31) transporters. In: Curr. Top. Membr. In: Bevens. Elsevier, Amsterdam, pp. 321–355
Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP (1999) Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285(5424):103–106
Takahashi N, Chernavvsky DR, Gomez RA, Igarashi P, Gitelman HJ, Smithies O (2000) Uncompensated polyuria in a mouse model of Bartter’s syndrome. Proc Natl Acad Sci U S A 97:5434–5439. doi:10.1073/pnas.090091297
Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, de Vegt F, D’Ancona FCH, den Heijer M, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K (2009) Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 41:926–930. doi:10.1038/ng.404
Toka HR, Genovese G, Mount DB, Pollak MR, Curhan GC (2013) Frequency of rare allelic variation in candidate genes among individuals with low and high urinary calcium excretion. PLoS One. doi:10.1371/journal.pone.0071885
Traglia M, Sala C, Masciullo C, Cverhova V, Lori F, Pistis G, Bione S, Gasparini P, Ulivi S, Ciullo M, Nutile T, Bosi E, Sirtori M, Mignogna G, Rubinacci A, Buetti I, Camaschella C, Petretto E, Toniolo D (2009) Heritability and demographic analyses in the large isolated population of val borbera suggest advantages in mapping complex traits genes. PLoS One 4:1–10. doi:10.1371/journal.pone.0007554
Tuschl K, Clayton PT, Gospe SM, Mills PB (2012) Dystonia/parkinsonism, hypermanganesemia, polycythemia, and chronic liver disease. In: Pragon RA et al. (ed) GeneReviews [Internet] Seattle (WA): University of Washington, Seattle; 1993–2016
Van Angelen AA, San-Cristobal P, Pulskens WP, Hoenderop JG, Bindels RJ (2013) The impact of dietary magnesium restriction on magnesiotropic and calciotropic genes. Nephrol Dial Transplant 28:2983–2993. doi:10.1093/ndt/gft358
Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CNA, Knott SA, Kolcic I, Polasek O, Graessler J, Wilson JF, Marinaki A, Riches PL, Shu X, Janicijevic B, Smolej-Narancic N, Gorgoni B, Morgan J, Campbell S, Biloglav Z, Barac-Lauc L, Pericic M, Klaric IM, Zgaga L, Skaric-Juric T, Wild SH, Richardson WA, Hohenstein P, Kimber CH, Tenesa A, Donnelly LA, Fairbanks LD, Aringer M, McKeigue PM, Ralston SH, Morris AD, Rudan P, Hastie ND, Campbell H, Wright AF (2008) SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 40:437–442. doi:10.1038/ng.106
Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, Belyantseva I, Ben-Yosef T, Liburd NA, Morell RJ, Kachar B, Wu DK, Griffith AJ, Riazuddin S, Friedman TB (2001) Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 104:165–172. doi:10.1016/S0092-8674(01)00200-8
Will C, Breiderhoff T, Thumfart J, Stuiver M, Kopplin K, Sommer K, Günzel D, Querfeld U, Meij IC, Shan Q, Bleich M, Willnow TE, Müller D, Günzel D, Querfeld U, Ic M, Shan Q, Bleich M (2010) Targeted deletion of murine Cldn16 identifies extra- and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am J Physiol Renal Physiol:1152–1161. doi:10.1152/ajprenal.00499.2009
Yu ASL (2015) Claudins and the kidney. J Am Soc Nephrol 26:11–19. doi:10.1681/ASN.2014030284
Acknowledgements
The CoLaus study is supported by research grants from GlaxoSmithKline, the Faculty of Biology and Medicine of Lausanne, and the Swiss National Science Foundation (grants 33CSCO-122661, 33CS30-139468, and 33CS30-148401). The computations for CoLaus imputation were performed in part at the Vital-IT Center for high performance computing of the Swiss Institute of Bioinformatics. MBO and TC are supported by the Swiss National Centre of Competence in Research Kidney Control of Homeostasis (NCCR Kidney.CH) program. OD is supported by grants from the European Community’s Seventh Framework Program (305608 EURenOmics), the Swiss National Centre of Competence in Research Kidney Control of Homeostasis (NCCR Kidney.CH) program, the Swiss National Science Foundation (310030-146490), and the Rare Disease Initiative Zurich (radiz), a clinical research priority program of the University of Zurich, Switzerland. EO is supported by the Fonds National de la Recherche Luxembourg (6903109) and the University Research Priority Program “Integrative Human Physiology, ZIHP” of the University of Zurich. NT is supported by funding from Swiss National Science Foundation Early and Advanced PostdocMolibity Fellowship (P2LAP3_151782 and P300P3_158521).
The CROATIA-Korcula and CROATIA-Split studies were funded by grants from the Medical Research Council (UK), European Commission Framework 6 project EUROSPAN (Contract No. LSHG-CT-2006-018947), and Republic of Croatia Ministry of Science, Education, and Sports research grants to IR (108-1080315-0302). We would like to acknowledge the invaluable contributions of the recruitment team in Korcula and Split, the administrative teams in Croatia and Edinburgh, and the people of Korcula and Split.
The SNP genotyping for the CROATIA-Korcula cohort was performed in Helmholtz Zentrum München, Neuherberg, Germany. The SNP genotyping for the CROATIA-Split cohort was performed by AROS Applied Biotechnology, Aarhus, Denmark.
INGI-Carlantino: We thank Anna Morgan and Angela D’Eustacchio for technical support. We are very grateful to the municipal administrators for their collaboration on the project and for logistic support. We would like to thank all participants to this study.
For the INGI-VALBORBERA study, the research was supported by funds from Compagnia di San Paolo, Torino, Italy; Fondazione Cariplo, Italy; and Ministry of Health, Ricerca Finalizzata 2008 to DT.
Phenotype collection in the Lothian Birth Cohort 1936 (LBC1936) was supported by Age UK (The Disconnected Mind project). Genotyping was funded by the BBSRC (BB/F019394/1). The work was undertaken by The University of Edinburgh Centre for Cognitive Aging and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative (MR/K026992/1). Funding from the BBSRC and Medical Research Council (MRC) is gratefully acknowledged. We thank the LBC1936 participants, the LBC1936 team for data collection and collation, and the staff at the Wellcome Trust Clinical Research Facility for bio-sample collection and genotyping.
Other funding sources: European Community’s Seventh Framework Program (FP7/2007–2013) under grant agreement no. 246539 (Marie Curie) and grant no. 305608 (EURenOmics), the NCCR Kidney.CH program (Swiss National Science Foundation), the Gebert Rüf Stiftung (Project GRS-038/12), and the Swiss National Science Foundation 310030-146490.
The authors acknowledge Nadine Nägele and Julien Weber for their help with the Platform of Biochemical Analyses at the University of Zurich and thank Jianghui Hou for the anti-claudin-14 antibodies and François Seghers, Yvette Cnops and Sébastien Druart (UCL Brussels) for help with the Mg2+ diets.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Additional information
Tanguy Corre and Eric Olinger jointly contributed
Caroline Hayward, Murielle Bochud, and Olivier Devuyst jointly directed the study
Electronic supplementary material
ESM 1
(DOCX 663 kb)
Rights and permissions
About this article
Cite this article
Corre, T., Olinger, E., Harris, S.E. et al. Common variants in CLDN14 are associated with differential excretion of magnesium over calcium in urine. Pflugers Arch - Eur J Physiol 469, 91–103 (2017). https://doi.org/10.1007/s00424-016-1913-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-016-1913-7