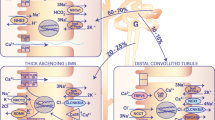
Abstract
The thick ascending limb of Henle’s loop (TAL) drives an important part of the reabsorption of divalent cations. This reabsorption occurs via the paracellular pathway formed by the tight junction (TJ), which in the TAL shows cation selectivity. Claudins, a family of TJ proteins, determine the permeability and selectivity of this pathway. Mice were fed with normal or high-Ca2+ diet, and effects on the reabsorptive properties of cortical and medullary TAL segments were analysed by tubule microdissection and microperfusion. Claudin expression was investigated by immunostaining and quantitative PCR. We show that the TAL adapted to high Ca2+ load in a sub-segment-specific manner. In medullary TAL, transcellular NaCl transport was attenuated. The transepithelial voltage decreased from 10.9 ± 0.6 mV at control diet to 8.3 ± 0.5 mV at high Ca2+ load, thereby reducing the driving force for Ca2+ and Mg2+ uptake. Cortical TAL showed a reduction in paracellular Ca2+ and Mg2+ permeabilities from 8.2 ± 0.7 to 6.2 ± 0.5 ∙ 10−4 cm/s and from 4.8 ± 0.5 to 3.0 ± 0.2 · 10−4 cm/s at control and high-Ca2+ diet, respectively. Expression, localisation and regulation of claudins 10, 14, 16 and 19 differed along the corticomedullary axis: Towards the cortex, the main site of divalent cation reabsorption in TAL, high-Ca2+ intake led to a strong upregulation of claudin-14 within TAL TJs while claudin-16 and -19 were unaltered. Towards the inner medulla, only claudin-10 was present in TAL TJ strands. In summary, high-Ca2+ diet induced a reduction of divalent cation reabsorption via a diminution of NaCl transport and driving force in mTAL and via decreased paracellular permeabilities in cTAL. We reveal an important regulatory pattern along the corticomedullary axis and improve the understanding how the kidney disposes of detrimental excess Ca2+.







Similar content being viewed by others
References
Bailly C, Imbert-Teboul M, Roinel N, Amiel C (1990) Isoproterenol increases Ca, Mg, and NaCl reabsorption in mouse thick ascending limb. Am J Physiol 258:F1224–1231
Barry PH, Lynch JW (1991) Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol 121:101–117
Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER, Rasmussen J, Anderson JM, Dolan DF, Forge A, Raphael Y, Camper SA, Friedman TB (2003) Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet 12:2049–2061
Bleich M, Shan Q, Himmerkus N (2012) Calcium regulation of tight junction permeability. Ann N Y Acad Sci 1258:93–99
Borschewski A, Himmerkus N, Boldt C, Blankenstein KI, McCormick JA, Lazelle R, Willnow TE, Jankowski V, Plain A, Bleich M, Ellison DH, Bachmann S, Mutig K (2015) Calcineurin and Sorting-Related Receptor with A-Type Repeats Interact to Regulate the Renal Na+−K+−2Cl- Cotransporter. J Am Soc Nephrol. doi:10.1681/ASN.2014070728
Breiderhoff T, Himmerkus N, Stuiver M, Mutig K, Will C, Meij IC, Bachmann S, Bleich M, Willnow TE, Müller D (2012) Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci U S A 109:14241–14246
de Rouffignac C, Quamme G (1994) Renal magnesium handling and its hormonal control. Physiol Rev 74:305–322
Di Stefano A, Roinel N, de Rouffignac C, Wittner M (1993) Transepithelial Ca2+ and Mg2+ transport in the cortical thick ascending limb of Henle's loop of the mouse is a voltage-dependent process. Ren Physiol Biochem 16:157–166
Di Stefano A, Wittner M, Nitschke R, Braitsch R, Greger R, Bailly C, Amiel C, Elalouf JM, Roinel N, de Rouffignac C (1989) Effects of glucagon on Na+, Cl-, K+, Mg2+ and Ca2+ transports in cortical and medullary thick ascending limbs of mouse kidney. Pflugers Arch 414:640–646
Di Stefano A, Wittner M, Nitschke R, Braitsch R, Greger R, Bailly C, Amiel C, Roinel N, de Rouffignac C (1990) Effects of parathyroid hormone and calcitonin on Na+, Cl-, K+, Mg2+ and Ca2+ transport in cortical and medullary thick ascending limbs of mouse kidney. Pflugers Arch 417:161–167
Dimke H, Desai P, Borovac J, Lau A, Pan W, Alexander RT (2013) Activation of the Ca(2+)-sensing receptor increases renal claudin-14 expression and urinary Ca(2+) excretion. Am J Physiol Renal Physiol 304:F761–769
Elkouby-Naor L, Abassi Z, Lagziel A, Gow A, Ben-Yosef T (2008) Double gene deletion reveals lack of cooperation between claudin 11 and claudin 14 tight junction proteins. Cell Tissue Res 333:427–438
Gamba G, Friedman PA (2009) Thick ascending limb: the Na(+):K (+):2Cl (−) co-transporter, NKCC2, and the calcium-sensing receptor, CaSR. Pflugers Arch 458:61–76
Gong Y, Himmerkus N, Plain A, Bleich M, Hou J (2015) Epigenetic regulation of microRNAs controlling CLDN14 expression as a mechanism for renal calcium handling. J Am Soc Nephrol 26:663–676
Gong Y, Hou J (2014) Claudin-14 underlies Ca(+)(+)-sensing receptor-mediated Ca(+)(+) metabolism via NFAT-microRNA-based mechanisms. J Am Soc Nephrol 25:745–760
Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J (2012) Claudin-14 regulates renal Ca(+)(+) transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 31:1999–2012
Greger R (1981) Cation selectivity of the isolated perfused cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch 390:30–37
Günzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M, Muller D (2009) Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci 122:1507–1517
Günzel D, Yu AS (2013) Claudins and the modulation of tight junction permeability. Physiol Rev 93:525–569
Hannan FM, Thakker RV (2013) Calcium-sensing receptor (CaSR) mutations and disorders of calcium, electrolyte and water metabolism. Best Pract Res Clin Endocrinol Metab 27:359–371
Hebert SC, Andreoli TE (1986) Ionic conductance pathways in the mouse medullary thick ascending limb of Henle. The paracellular pathway and electrogenic Cl- absorption. J Gen Physiol 87:567–590
Himmerkus N, Shan Q, Goerke B, Hou J, Goodenough DA, Bleich M (2008) Salt and acid–base metabolism in claudin-16 knockdown mice: impact for the pathophysiology of FHHNC patients. Am J Physiol Renal Physiol 295:F1641–1647
Hoek AC, Lemmens AG, Mullink JW, Beynen AC (1988) Influence of dietary calcium:phosphorus ratio on mineral excretion and nephrocalcinosis in female rats. J Nutr 118:1210–1216
Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA (2009) Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A 106:15350–15355
Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, Bleich M, Goodenough DA (2007) Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem 282:17114–17122
Kimizuka H, Koketsu K (1964) Ion transport through cell membrane. J Theor Biol 6:290–305
Kirk A, Campbell S, Bass P, Mason J, Collins J (2010) Differential expression of claudin tight junction proteins in the human cortical nephron. Nephrol Dial Transplant 25:2107–2119
Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S (2002) Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13:875–886
Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S (2006) Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 79:949–957
Loupy A, Ramakrishnan SK, Wootla B, Chambrey R, de la Faille R, Bourgeois S, Bruneval P, Mandet C, Christensen EI, Faure H, Cheval L, Laghmani K, Collet C, Eladari D, Dodd RH, Ruat M, Houillier P (2012) PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J Clin Invest 122:3355–3367
Matsubara M (2004) Renal sodium handling for body fluid maintenance and blood pressure regulation. Yakugaku Zasshi 124:301–309
Ng B, Barry PH (1995) The measurement of ionic conductivities and mobilities of certain less common organic ions needed for junction potential corrections in electrophysiology. J Neurosci Methods 56:37–41
Ohta H, Adachi H, Takiguchi M, Inaba M (2006) Restricted localization of claudin-16 at the tight junction in the thick ascending limb of Henle's loop together with claudins 3, 4, and 10 in bovine nephrons. J Vet Med Sci 68:453–463
Peterson CA, Baker DH, Erdman JW Jr (1996) Diet-induced nephrocalcinosis in female rats is irreversible and is induced primarily before the completion of adolescence. J Nutr 126:259–265
Schaafsma G, Duursma SA, Visser WJ, Dekker PR (1985) The influence of dietary calcium on kidney calcification and renal function in rats fed high-phosphate diets. Bone 6:155–163
Shan Q, Himmerkus N, Hou J, Goodenough DA, Bleich M (2009) Insights into driving forces and paracellular permeability from claudin-16 knockdown mouse. Ann N Y Acad Sci 1165:148–151
Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP (1999) Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285:103–106
Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, de Vegt F, d'Ancona FC, den Heijer M, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K (2009) Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 41:926–930
Toka HR, Pollak MR, Houillier P (2015) Calcium Sensing in the Renal Tubule. Physiology (Bethesda) 30:317–326
Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM (2006) Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol 291:F1288–1299
Will C, Breiderhoff T, Thumfart J, Stuiver M, Kopplin K, Sommer K, Günzel D, Querfeld U, Meij IC, Shan Q, Bleich M, Willnow TE, Müller D (2010) Targeted deletion of murine Cldn16 identifies extra- and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am J Physiol Renal Physiol 298:F1152–1161
Wittner M, di Stefano A, Wangemann P, Nitschke R, Greger R, Bailly C, Amiel C, Roinel N, de Rouffignac C (1988) Differential effects of ADH on sodium, chloride, potassium, calcium and magnesium transport in cortical and medullary thick ascending limbs of mouse nephron. Pflugers Arch 412:516–523
Acknowledgments
We thank Kerim Mutig (Charité, Berlin) for kindly providing the NKCC2 antibody.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was funded by the Christian-Albrechts-University Kiel.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Primers applied for Real Time PCR (DOC 29 kb)
Rights and permissions
About this article
Cite this article
Plain, A., Wulfmeyer, V.C., Milatz, S. et al. Corticomedullary difference in the effects of dietary Ca2+ on tight junction properties in thick ascending limbs of Henle’s loop. Pflugers Arch - Eur J Physiol 468, 293–303 (2016). https://doi.org/10.1007/s00424-015-1748-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-015-1748-7