Abstract
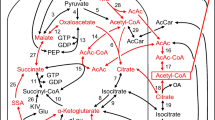
Pancreatic beta-cells respond to an unchanging stimulatory glucose concentration with oscillations in membrane potential (Vm), cytosolic Ca2+ concentration ([Ca2+]c), and insulin secretion. The underlying mechanisms are largely ascertained. Some particular details, however, are still in debate. Stimulus-secretion coupling (SSC) of beta-cells comprises glucose-induced Ca2+ influx into the cytosol and thus into mitochondria. It is suggested that this activates (mitochondrial) dehydrogenases leading to an increase in reduction equivalents and ATP production. According to SSC, a glucose-induced increase in ATP production would thus further augment ATP production, i.e. induce a feed-forward loop that is hardly compatible with oscillations. Consistently, other studies favour a feedback mechanism that drives oscillatory mitochondrial ATP production. If Ca2+ influx activates dehydrogenases, a change in [Ca2+]c should increase the concentration of reduction equivalents. We measured changes in flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide (phosphate) (NAD(P)H) autofluorescence in response to changes in glucose concentration or glucose-independent changes in [Ca2+]c. The FAD signal was altered by glucose but not by alterations in [Ca2+]c. NAD(P)H was increased by glucose but even decreased by Ca2+ influx evoked by tolbutamide. The mitochondrial membrane potential ΔΨ was hyperpolarized by 4 mM glucose. As adding tolbutamide then depolarized ΔΨ, we deduce that Ca2+ does not activate mitochondrial activity but by contrast even inhibits it by reducing the driving force for ATP production. Inhibition of Ca2+ influx reversed the Ca2+-induced changes in ΔΨ and NAD(P)H. The results are consistent with a feedback mechanism which transiently and repeatedly reduces ATP production and explain the oscillatory activity of pancreatic beta-cells at increased glucose concentrations.





Similar content being viewed by others
References
Alam MR, Groschner LN, Parichatikanond W, Kuo L, Bondarenko AI, Rost R, Waldeck-Weiermair M, Malli R, Graier WF (2012) Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial Ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic beta-cells. J Biol Chem 287(41):34445–34454. doi:10.1074/jbc.M112.392084
Belz M, Willenborg M, Gorgler N, Hamada A, Schumacher K, Rustenbeck I (2014) Insulinotropic effect of high potassium concentration beyond plasma membrane depolarization. Am J Physiol Endocrinol Metab 306(6):E697–706. doi:10.1152/ajpendo.00362.2013
Bergsten P, Grapengiesser E, Gylfe E, Tengholm A, Hellman B (1994) Synchronous oscillations of cytoplasmic Ca2+ and insulin release in glucose-stimulated pancreatic islets. J Biol Chem 269(12):8749–8753
Brandman O, Meyer T (2008) Feedback loops shape cellular signals in space and time. Science 322(5900):390–395
Civelek VN, Deeney JT, Shalosky NJ, Tornheim K, Hansford RG, Prentki M, Corkey BE (1996) Regulation of pancreatic beta-cell mitochondrial metabolism: influence of Ca2+, substrate and ADP. Biochem J 318(Pt 2):615–621
De Marchi U, Thevenet J, Hermant A, Dioum E, Wiederkehr A (2014) Calcium co-regulates oxidative metabolism and ATP synthase-dependent respiration in pancreatic beta cells. J Biol Chem 289(13):9182–9194
Dean PM, Matthews EK (1968) Electrical activity in pancreatic islet cells. Nature 219(5152):389–390
Denton RM (2009) Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787(11):1309–1316. doi:10.1016/j.bbabio.2009.01.005
Detimary P, Dejonghe S, Ling Z, Pipeleers D, Schuit F, Henquin JC (1998) The changes in adenine nucleotides measured in glucose-stimulated rodent islets occur in beta cells but not in alpha cells and are also observed in human islets. J Biol Chem 273(51):33905–33908
Detimary P, Gilon P, Henquin JC (1998) Interplay between cytoplasmic Ca2+ and the ATP/ADP ratio: a feedback control mechanism in mouse pancreatic islets. Biochem J 333(Pt 2):269–274
Drews G, Krippeit-Drews P, Düfer M (2015) Electrophysiology of islet cells. In: Islam MS (ed) Islets of Langerhans, vol 1, 2nd edn. Springer Dordrecht, Heidelberg, pp 249–303. doi:10.1007/978-94-007-6686-0_5
Duchen MR, Smith PA, Ashcroft FM (1993) Substrate-dependent changes in mitochondrial function, intracellular free calcium concentration and membrane channels in pancreatic beta-cells. Biochem J 294(Pt 1):35–42
Düfer M, Gier B, Wolpers D, Krippeit-Drews P, Ruth P, Drews G (2009) Enhanced glucose tolerance by SK4 channel inhibition in pancreatic beta-cells. Diabetes 58:1835–1843
Düfer M, Haspel D, Krippeit-Drews P, Kelm M, Ranta F, Nitschke R, Ullrich S, Aguilar-Bryan L, Bryan J, Drews G (2007) The KATP channel is critical for calcium sequestration into non-ER compartments in mouse pancreatic beta cells. Cell Physiol Biochem 20(1–4):65–74
Düfer M, Noack K, Krippeit-Drews P, Drews G (2010) Activation of the AMP-activated protein kinase enhances glucose-stimulated insulin secretion in mouse beta-cells. Islets 2(3):156–163
Dyachok O, Isakov Y, Sagetorp J, Tengholm A (2006) Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature 439(7074):349–352. doi:10.1038/nature04410
Gilon P, Shepherd RM, Henquin JC (1993) Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidences in single pancreatic islets. J Biol Chem 268(30):22265–22268
Goehring I, Gerencser AA, Schmidt S, Brand MD, Mulder H, Nicholls DG (2012) Plasma membrane potential oscillations in insulin secreting Ins-1 832/13 cells do not require glycolysis and are not initiated by fluctuations in mitochondrial bioenergetics. J Biol Chem 287(19):15706–15717
Göpel SO, Kanno T, Barg S, Eliasson L, Galvanovskis J, Renstrom E, Rorsman P (1999) Activation of Ca(2+)-dependent K(+) channels contributes to rhythmic firing of action potentials in mouse pancreatic beta cells. J Gen Physiol 114(6):759–770
Grapengiesser E, Gylfe E, Hellman B (1988) Dual effect of glucose on cytoplasmic Ca2+ in single pancreatic beta-cells. Biochem Biophys Res Commun 150(1):419–425
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260(6):3440–3450
Hagren OI, Tengholm A (2006) Glucose and insulin synergistically activate phosphatidylinositol 3-kinase to trigger oscillations of phosphatidylinositol 3,4,5-trisphosphate in beta-cells. J Biol Chem 281(51):39121–39127. doi:10.1074/jbc.M607445200
Henquin JC (1990) Cellular mechanisms of the control of insulin secretion. Arch Int Physiol Biochim 98(3):A61–80
Jung SK, Kauri LM, Qian WJ, Kennedy RT (2000) Correlated oscillations in glucose consumption, oxygen consumption, and intracellular free Ca(2+) in single islets of Langerhans. J Biol Chem 275(9):6642–6650
Kennedy ED, Rizzuto R, Theler JM, Pralong WF, Bastianutto C, Pozzan T, Wollheim CB (1996) Glucose-stimulated insulin secretion correlates with changes in mitochondrial and cytosolic Ca2+ in aequorin-expressing INS-1 cells. J Clin Invest 98(11):2524–2538. doi:10.1172/JCI119071
Kennedy ED, Wollheim CB (1998) Role of mitochondrial calcium in metabolism-secretion coupling in nutrient-stimulated insulin release. Diabetes Metab 24(1):15–24
Kennedy RT, Kauri LM, Dahlgren GM, Jung SK (2002) Metabolic oscillations in beta-cells. Diabetes 51(Suppl 1):S152–161
Kindmark H, Köhler M, Brown G, Bränström R, Larsson O, Berggren PO (2001) Glucose-induced oscillations in cytoplasmic free Ca2+ concentration precede oscillations in mitochondrial membrane potential in the pancreatic beta-cell. J Biol Chem 276(37):34530–34536. doi:10.1074/jbc.M102492200
Köhler M, Norgren S, Berggren PO, Fredholm BB, Larsson O, Rhodes CJ, Herbert TP, Luthman H (1998) Changes in cytoplasmic ATP concentration parallels changes in ATP-regulated K+-channel activity in insulin-secreting cells. FEBS Lett 441(1):97–102
Krippeit-Drews P, Düfer M, Drews G (2000) Parallel oscillations of intracellular calcium activity and mitochondrial membrane potential in mouse pancreatic B-cells. Biochem Biophys Res Commun 267(1):179–183. doi:10.1006/bbrc.1999.1921
Li J, Shuai HY, Gylfe E, Tengholm A (2013) Oscillations of sub-membrane ATP in glucose-stimulated beta cells depend on negative feedback from Ca(2+). Diabetologia 56(7):1577–1586. doi:10.1007/s00125-013-2894-0
Longo EA, Tornheim K, Deeney JT, Varnum BA, Tillotson D, Prentki M, Corkey BE (1991) Oscillations in cytosolic free Ca2+, oxygen consumption, and insulin secretion in glucose-stimulated rat pancreatic islets. J Biol Chem 266(14):9314–9319
Luciani DS, Misler S, Polonsky KS (2006) Ca2+ controls slow NAD(P)H oscillations in glucose-stimulated mouse pancreatic islets. J Physiol 572(Pt 2):379–392
MacDonald MJ, Brown LJ (1996) Calcium activation of mitochondrial glycerol phosphate dehydrogenase restudied. Arch Biochem Biophys 326(1):79–84. doi:10.1006/abbi.1996.0049
Malaisse WJ, Sener A (1991) Hexose metabolism in pancreatic islets. Activation of the Krebs cycle by nutrient secretagogues. Mol Cell Biochem 107(2):95–102
McCormack JG, Longo EA, Corkey BE (1990) Glucose-induced activation of pyruvate dehydrogenase in isolated rat pancreatic islets. Biochem J 267(2):527–530
Meissner HP, Schmidt H (1976) The electrical activity of pancreatic beta-cells of diabetic mice. FEBS Lett 67(3):371–374
Rolland JF, Henquin JC, Gilon P (2002) Feedback control of the ATP-sensitive K+ current by cytosolic Ca2+ contributes to oscillations of the membrane potential in pancreatic beta-cells. Diabetes 51(2):376–384
Rorsman P, Braun M (2013) Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 75:155–179. doi:10.1146/annurev-physiol-030212-183754
Rorsman P, Eliasson L, Kanno T, Zhang Q, Gopel S (2011) Electrophysiology of pancreatic beta-cells in intact mouse islets of Langerhans. Prog Biophys Mol Biol 107(2):224–235
Rorsman P, Eliasson L, Renstrom E, Gromada J, Barg S, Gopel S (2000) The cell physiology of biphasic insulin secretion. News Physiol Sci 15:72–77
Santos RM, Rosario LM, Nadal A, Garcia-Sancho J, Soria B, Valdeolmillos M (1991) Widespread synchronous [Ca2+]i oscillations due to bursting electrical activity in single pancreatic islets. Pflugers Arch 418(4):417–422
Schulze DU, Düfer M, Wieringa B, Krippeit-Drews P, Drews G (2007) An adenylate kinase is involved in KATP channel regulation of mouse pancreatic beta cells. Diabetologia 50(10):2126–2134. doi:10.1007/s00125-007-0742-9
Sener A, Rasschaert J, Malaisse WJ (1990) Hexose metabolism in pancreatic islets. Participation of Ca2(+)-sensitive 2-ketoglutarate dehydrogenase in the regulation of mitochondrial function. Biochim Biophys Acta 1019(1):42–50
Shuttleworth CW, Brennan AM, Connor JA (2003) NAD(P)H fluorescence imaging of postsynaptic neuronal activation in murine hippocampal slices. J Neurosci 23(8):3196–3208
Tarasov AI, Girard CA, Ashcroft FM (2006) ATP sensitivity of the ATP-sensitive K+ channel in intact and permeabilized pancreatic beta-cells. Diabetes 55(9):2446–2454. doi:10.2337/db06-0360
Tarasov AI, Semplici F, Li D, Rizzuto R, Ravier MA, Gilon P, Rutter GA (2013) Frequency-dependent mitochondrial Ca(2+) accumulation regulates ATP synthesis in pancreatic beta cells. Pflugers Arch 465(4):543–554. doi:10.1007/s00424-012-1177-9
Thore S, Dyachok O, Tengholm A (2004) Oscillations of phospholipase C activity triggered by depolarization and Ca2+ influx in insulin-secreting cells. J Biol Chem 279(19):19396–19400. doi:10.1074/jbc.C400088200
Acknowledgments
The work was supported by grants of the Deutsche Forschungsgemeinschaft (G.D., M.D.) and the Deutsche Diabetes-Stiftung (G.D.). We thank Prof. J. Bryan for fruitful discussions about the manuscript.
Compliance with Ethical Standards
ᅟ
Informed consent
Not applicable
Research involving human participants and/or animals
Experiments were performed using organs from adult mice. The principles of laboratory animal care (Guide for the care and use of laboratory animals, NIH publication no. 85–23, revised 1985) and German laws were followed
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drews, G., Bauer, C., Edalat, A. et al. Evidence against a Ca2+-induced potentiation of dehydrogenase activity in pancreatic beta-cells. Pflugers Arch - Eur J Physiol 467, 2389–2397 (2015). https://doi.org/10.1007/s00424-015-1707-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-015-1707-3