Abstract
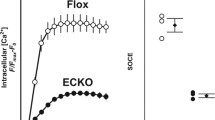
We determined the in vivo role of stromal-interacting molecule 1 (STIM1) in the regulation of vascular function using endothelial cell (EC)- and smooth-muscle (SM)-specific knockout mice. Systolic blood pressure and glucose levels were similar in all mice (Stim1SMC−/−, Stim1SMC−/+, Stim1EC−/−, Stim1EC−/+), but body weight was reduced in Stim1EC−/− and Stim1SMC−/− mice. The contraction of arteries in response to phenylephrine was significantly reduced in Stim1SMC−/− mice only. However, contraction to thromboxane and KCl was similar in all groups. The endothelium-dependent relaxation (EDR) was impaired in Stim1EC−/+ and drastically reduced in Stim1EC−/− mice while the endothelium-independent vasorelaxation was similar among all groups. Acute downregulation of STIM1 in arteries reduced EDR and the contractile response to phenylephrine, while the contractile response to thromboxane was not affected. NADPH oxidase activity was increased only in Stim1EC−/+ and Stim1EC−/− mice. Calcium (Ca2+) entry in endothelial cells stimulated with thrombin and histamine had the pharmacological features of store-operated Ca2+ entry (SOCE) and was dependent on STIM1 expression. We conclude that STIM1 plays opposing roles in vascular smooth muscle vs. endothelial cells in the regulation of vascular reactivity.





Similar content being viewed by others
References
Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M (2008) Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res 103:1289–1299. doi:10.1161/01
Aird WC (2008) Endothelium in health and disease. Pharmacol Rep 60:139–143
Aubart FC, Sassi Y, Coulombe A, Mougenot N, Vrignaud C, Leprince P, Lechat P, Lompré AM, Hulot JS (2009) RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol Ther 17:455–462. doi:10.1038/mt.2008.291
Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Matrougui K (2008) Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes 57:1629–1637. doi:10.2337/db07-0739
Choi SK, Galán M, Kassan M, Partyka M, Trebak M, Matrougui K (2012) Poly(ADP-ribose) polymerase 1 inhibition improves coronary arteriole function in type 2 diabetes mellitus. Hypertension 59:1060–1068. doi:10.1161/HYPERTENSIONAHA.111.190140
Domínguez-Rodríguez A, Díaz I, Rodríguez-Moyano M, Calderón-Sánchez E, Rosado JA, Ordóñez A, Smani T (2012) Urotensin-II signaling mechanism in rat coronary artery: role of STIM1 and Orai1-dependent store operated calcium influx in vasoconstriction. Arterioscler, Thromb, Vasc Biol 32:1325–1332. doi:10.1161/ATVBAHA.111.243014
Estrada IA, Donthamsetty R, Debski P, Zhou MH, Zhang SL, Yuan JX, Han W, Makino A (2012) STIM1 restores coronary endothelial function in type 1 diabetic mice. Circ Res 111:1166–1175. doi:10.1161/CIRCRESAHA.112.275743
Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, Razmpour R, Yang XF, Houser SR, Chen J, Koch WJ, Wang H, Soboloff J, Gill DL, Madesh M (2013) Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest 123:887–902. doi:10.1172/JCI65647
Giachini FR, Chiao CW, Carneiro FS, Lima VV, Carneiro ZN, Dorrance AM, Tostes RC, Webb RC (2009) Increased activation of stromal interaction molecule-1/Orai-1 in aorta from hypertensive rats: a novel insight into vascular dysfunction. Hypertension 53:409–416. doi:10.1161/HYPERTENSIONAHA.108.124404
Guo RW, Wang H, Gao P, Li MQ, Zeng CY, Yu Y, Chen JF, Song MB, Shi YK, Huang L (2009) An essential role for stromal interaction molecule 1 in neointima formation following arterial injury. Cardiovasc Res 8:660–668. doi:10.1093/cvr/cvn338
House SJ, Potier M, Bisaillon J, Singer HA, Trebak M (2008) The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch 456:769–785. doi:10.1007/s00424-008-0491-8
Kassan M, Choi SK, Galán M, Bishop A, Umezawa K, Trebak M, Belmadani S, Matrougui K (2013) Enhanced NF-κB activity impairs vascular function through PARP-1-, SP-1-, and COX-2-dependent mechanisms in type 2 diabetes. Diabetes 62:2078–2087. doi:10.2337/db12-1374
Kassan M, Galán M, Partyka M, Saifudeen Z, Henrion D, Trebak M, Matrougui K (2012) Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler, Thromb, Vasc Biol 32:1652–1661. doi:10.1161/ATVBAHA.112.249318
Mancarella S, Potireddy S, Wang Y, Gao H, Gandhirajan RK, Autieri M, Scalia R, Cheng Z, Wang H, Madesh M, Houser SR, Gill DL (2013) Targeted STIM deletion impairs calcium homeostasis, NFAT activation, and growth of smooth muscle. FASEB J 27:893–906. doi:10.1096/fj.12-215293
Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A (2008) Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol 9:432–443. doi:10.1038/ni1574
Pittman RN (2011) San Rafael (CA): Morgan & Claypool Life Sciences
Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M (2009) Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J 23:2425–2437. doi:10.1096/fj.09-131128
Ruhle B, Trebak M (2013) Emerging roles for native Orai Ca2+ channels in cardiovascular disease. Curr Top Membr 71:209–235. doi:10.1016/B978-0-12-407870-3.00009-3
Shinde AV, Motiani RK, Zhang X, Abdullaev IF, Adam AP, González-Cobos JC, Zhang W, Matrougui K, Vincent PA, Trebak M (2013) STIM1 controls endothelial barrier function independently of Orai1 and Ca2+ entry. Sci Signal ra18. doi: 10.1126/scisignal.2003425
Voelkers M, Salz M, Herzog N, Frank D, Dolatabadi N, Frey N, Gude N, Friedrich O, Koch WJ, Katus HA, Sussman MA, Most P (2010) Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol 48:1329–1334. doi:10.1016/j.yjmcc.2010.01.020
Zbidi H, López JJ, Amor NB, Bartegi A, Salido GM, Rosado JA (2009) Enhanced expression of STIM1/Orai1 and TRPC3 in platelets from patients with type 2 diabetes mellitus. Blood Cells Mol Dis 43:211–213. doi:10.1016/j.bcmd.2009.04.005
Zhang J, Zhong W, Cui T, Yang M, Hu X, Xu K, Xie C, Xue C, Gibbons GH, Liu C, Li L, Chen YE (2006) Generation of an adult smooth muscle cell-targeted Cre recombinase mouse model. Arterioscler, Thromb, Vasc Biol 26:e23–e24
Zhang W, Halligan KE, Zhang X, Bisaillon JM, Gonzalez-Cobos JC, Motiani RK, Hu G, Vincent PA, Zhou J, Barroso M, Singer HA, Matrougui K, Trebak M (2011) Orai1-mediated I (CRAC) is essential for neointima formation after vascular injury. Circ Res 109:534–542. doi:10.1161/CIRCRESAHA.111.246777
Sources of funding
This work was supported by the NIH (HL095566 to KM and HL097111 to MT), AHA grants (14GRNT18880008 to MT and 16850060 to MK), and Applied Biophysics Inc. Troy, NY to JS.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Modar Kassan and Wei Zhang have equally contributed to this work.
Khalid Matrougui and Mohamed Trebak share senior authorship.
Rights and permissions
About this article
Cite this article
Kassan, M., Zhang, W., Aissa, K.A. et al. Differential role for stromal interacting molecule 1 in the regulation of vascular function. Pflugers Arch - Eur J Physiol 467, 1195–1202 (2015). https://doi.org/10.1007/s00424-014-1556-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1556-5