Abstract
Background and objectives
Mean survival time (MST) is used as the indicator of prognosis in patients with a colorectal cancer (CRC) recurrence. The present study aimed to visualize the changes in death risk after a CRC recurrence using hazard function analysis (HFA) to provide an alternative prognostic indicator to MST.
Methods
The medical records of 725 consecutive patients with a recurrence following R0 radical surgery for CRC were retrospectively reviewed.
Results
The five-year, post-recurrence survival rate was 37.8%, and the MST was 3.5 years while the risk of death peaked at 2.9 years post-recurrence. Seven variables were found to predict short-term survival, including the number of metastatic organs ≥ 2, non-surgical treatment for the recurrence, and a short interval before recurrence. In patients with a recurrence in one organ, the MST was four years, the peak time of death predicted by HFA was 2.9 years, and the five-year survival rate was 45.8%. In patients with a surgical resection of the recurrence, the MST was 8 years, the peak time of death was 3.3 years, and the five-year survival rate was 62%.
Conclusions
The present study established a novel method of assessing changes in mortality risk over time using HFA in patients with a CRC recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is one of the most common, malignant diseases and the second most frequent cause of cancer-related death worldwide [1]. The cumulative recurrence rate is reportedly around 20% [2], and surgical resection is the standard treatment if the recurrence is curatively resectable. In cases where surgical treatment is unsuitable, systemic chemotherapy may be considered; owing to recent advances in chemotherapy, the median survival time (MST) post-recurrence has lengthened to more than 30 months [3]. However, despite these treatments, a large proportion of patients with colorectal cancer recurrence still die from the disease. Therefore, it is important to predict the survival outcome whenever a recurrence is observed. The Kaplan–Meier survival estimate is the standard method of assessing survival outcomes in clinical studies [4], and the median survival time (MST), or the period during which 50% of patients in a given population die according to the Kaplan–Meier estimate, is generally used as the standard index of patient prognosis [5]. However, in the daily clinical setting, the MST should not be considered an ideal prognostic indicator because a patient with a recurrence really wants to know two things: “What is the probability of my survival?” and “If I can’t survive the recurrence, how long can I survive?” The second question can be paraphrased as, “When does my risk of death peak?” The answers to these questions cannot be found in the MST alone; rather, the focus needs to be placed on two survival indicators, the five-year survival probability and an assessment of changes in the hazard rate of death over time using hazard function analysis (HFA). HFA, a method of analysis proposed by Simes and Zelen, plots data with the horizontal axis representing time and the vertical axis representing the hazard rate [6]. HFA enables the risk of an event, such as death or the recurrence peak [4, 6], to be visualized. Cancer conditions at the time of a recurrence vary widely between individuals from a surgically resectable, single recurrence in the liver to multiple, unresectable metastases in multiple organs. The resulting prognosis accordingly varies widely. The present study aimed to demonstrate improved prediction of the prognosis by calculating the five-year survival probability and the most frequent time of death after recurrence in each of the patients enrolled.
Methods
Patients
The medical records of 725 consecutive patients with a CRC recurrence who had undergone surgery with curative intent between January 2005 and December 2016 in the Department of Surgery at Tokyo Metropolitan Cancer and Infectious Diseases Center at Komagome Hospital were retrospectively reviewed. The right-sided colon was defined as the bowel proximal to the splenic flexure consisting of the cecum and the ascending and transverse colon, and the left-sided colon was defined as the bowel distal to the splenic flexure consisting of the descending and sigmoid colon and rectum. Tumor staging was performed using the TNM classification of malignant tumors, 8th edition [7]. All the patients had undergone postoperative surveillance according to the surveillance protocol defined by the Japanese Society for Cancer of the Colon and Rectum guidelines [2]. Tumor markers (carcinoembryonic antigen, carbohydrate antigen 19–9) were examined every three months, computed tomography (CT) was performed every six months, and a total colonoscopy was recommended after the first postoperative year and every two to three years thereafter.
A recurrence was diagnosed on the basis of biopsy, MRI, PET/CT or CT findings at follow-up. The recurrence date was recorded as the date of diagnosis of the first recurrence. The present study was approved by the institutional review board of the study center (reference number: 3080).
Statistical analysis
Variables with P < 0.05 on univariate analysis were subjected to multivariate Cox proportional hazards analysis. The five-year cumulative incidence of recurrence was calculated using the Kaplan–Meier method. Survival estimate was made using JMP Pro 16.2 (SAS Institute Inc., Cary, NC, USA), and hazard function analysis was performed using R 3.6.1 with the muhaz package (R Project for Statistical Computing, Vienna, Austria; http://www.r-project.org/).
Results
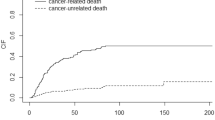
Table 1 shows the general characteristics of the patients included in this study. The most frequent site of the initial cancer was the rectum (54.9%). Adjuvant chemotherapy was administered in 43% of the patients. The most frequent site of recurrence was the liver (37.8%) followed by the lung (31.9%) and lymph node (23.2%). The median interval between the initial surgery and the recurrence was 1.0 year, and the median follow-up period from the recurrence was 3.4 years. The five-year survival rate after a recurrence was 37.8%, and the MST was 3.5 years (Fig. 1a). The risk of death peaked at 2.9 years post-recurrence (Fig. 1b).
Table 2 shows the results of univariate and multivariate analyses of the association between variables and post-recurrence survival. Based on the results, the following seven variables were found to predict shorter survival: age ≥ 60 years, T4, N ≥ 1, histological type other than well or moderately differentiated adenocarcinoma, number of metastatic organs ≥ 2, non-surgical treatment for the recurrence, and interval between initial surgery and the recurrence < 2 years.
Changes in the hazard rate of death with the passage of time in the seven variables were compared (Fig. 2). The older patient group had a shorter peak time (2.79 years) than the younger group (3.03 years) (Fig. 2a). The hazard rate was higher in T4 patients (0.342) than in T1/2/3 patients (0.183, 0.194, 0.178, respectively), peaking at 3.11 years after recurrence (Fig. 2b). The hazard rate was higher in N2 patients (0.350) than in N0/1 patients (0.147, 0.226), peaking at 2.86 years (Fig. 2c). Histological types other than well or moderately differentiated adenocarcinoma were associated with a greater risk of death, which was especially high in the early period after the detection of a recurrence (Fig. 2d). The hazard rate increased as the number of metastatic organs increased. If a metastasis was found in only one organ, the peak hazard rate was 0.185; if a metastasis was found in two organs, it increased to 0.465. For three or more organs, the hazard rate increased to 0.702 (Fig. 2e). The hazard rate was lower in patients with surgery for the initial recurrence (0.130) than in those with chemotherapy, best supportive care, etc. (0.443, 1.186, and 0.350, respectively) (Fig. 2f). The hazard rate was higher (0.245) if the interval between surgery and recurrence was < 2 years than if it was longer (0.174) and peaked at 2.85 years (Fig. 2g).
Differences in risk of death by prognostic factor. Hazard function plots stratified by (a) age (black line, < 60 years old; red line, ≥ 60 years old), b T stage (black line, T1; blue line, T2; red line, T3; green line, T4), c N stage (black line, N0; blue line, N1; red line, N2), d histological type (black line, well or moderately differentiated adenocarcinoma; red line, others), e number of metastatic organs (black line, 1; blue line, 2; red line, ≥ 3), f treatment for recurrence (black line, chemotherapy; blue line, surgery; red line, best-supported care; green line, others), and (g) interval between initial surgery and recurrence (black line, ≥ 2 years; red line, < 2 years)
The MST, five-year survival after recurrence, and peak time of death were compared according to the number of metastatic organs and treatment for the recurrence (Table 3). If the recurrence was confined one organ, the MST was four years whereas the peak time of death was 2.9 years, and 45.8% of the patients survived five years post-recurrence. The MST of the patients with surgery for a recurrence was eight years, their peak time of death was 3.3 years, and 62% survived five years post-recurrence (Table 3).
Discussion
The present study is the first to investigate survival after a colorectal cancer recurrence using HFA. A previous study reported that the incidence of recurrence by organ was 35.5%, 23.8%, and 19.9% for the liver, lung, and local organ, respectively [8], which was in line with the present findings.
The patients’ question, “How long can I survive?” should be a combination of two questions, “How likely am I to die from cancer?” and “If I am to die, when?”. The answer to the former question should be the 5-year survival, and the answer to the latter question should be the peak time determined by hazard function analysis. Figure 1 shows that around 60% of the patients died within five years of a recurrence, and HFA found a peak time of three years, indicating that death after the diagnosis of a recurrence occurred most frequently around three years post-recurrence.
Seven factors were found to be independent variables of survival post-recurrence: age, T stage, N stage, histological type, number of metastatic organs, treatment, and the interval between the initial surgery and the recurrence. Among these, treatment and the number of metastatic organs were the most important as indicated by their high hazard ratio. Several studies have demonstrated that right-sided colon cancer, histological type other than well or moderately differentiated adenocarcinoma, lymph node metastasis, presence of peritoneal metastasis at the time of recurrence, recurrence in ≥ 2 organs, surgically unresectable recurrence, and recurrence within postoperative 2 years [9, 10] were indicative of shorter survival after a colorectal cancer recurrence, in line with the findings of the present study.
Although old age and a short interval until recurrence were both risk factors of poor survival, it is unclear whether the death rate was high or the survival time was short. In the present study, HFA demonstrated that the death risk was higher during the first three years after a recurrence in the elderly patient group and the shorter interval group but converged thereafter (Fig. 2a, g). This may be explained by the less intensive treatment given to the elderly patients because of the presence of comorbidities and their impaired performance status [11, 12] and by rapid tumor growth in the shorter interval group [13,14,15]. Ryuk et al. reported that the five-year survival rate of colorectal cancer patients with a recurrence within two years after primary tumor resection was 34.7% while in patients with a longer interval it was 78.8%, corroborating our findings [14].
A higher stage of the primary tumor was also associated with poor prognosis post-recurrence. The hazard rate remained higher in T4 than T1 to T3 and in N2 than in N0 or N1 throughout the entire period probably because of higher invasiveness and metastatic potential (Fig. 2b, c). The peak time in all the groups was around three years, with the difference being non-significant. Connell et al. reported that the stage of the primary tumor was also an independent prognostic factor of tumor recurrence [16] as seen in the present study.
Patients with a histological type other than well or moderately differentiated adenocarcinoma mostly had poorly differentiated or mucinous adenocarcinoma and extremely short, post-recurrence survival (Fig. 2d). Their hazard rate peaked markedly within one year post-recurrence, suggesting that most patients with a recurrence of one of these histological types died within one year post-recurrence despite the frequency of the histological types being only around 10% [17]. Among the histological types in question, poorly differentiated adenocarcinoma is known to be especially malignant owing to an accumulation of genetic alterations and higher proliferative potential [18, 19] and also is reportedly less sensitive to chemotherapy than other types [20, 21].
As the number of metastatic organs increased, the hazard rate also increased possibly because R0 resection tended to be unfeasible in cases of multiple organ metastasis, and also because carcinomas with metastases to more than one organ may be more aggressive (Fig. 2e). Previous studies have also reported that the number of metastatic organs is also an important prognostic factor [9, 22].
The hazard ratio was higher in patients receiving chemotherapy than in those with recurrence resection throughout the whole period, with the ratio plateauing from two to five years post-recurrence without a clear peak. This suggests that most patients were able to survive about two years with chemotherapy alone but began dying during the following three years, a finding which conforms closely with clinical experience.
Table 3 shows that the MST should not be treated as a reliable indicator of survival. For instance, in patients with a recurrence in only one organ, the five-year survival rate is 45.8%, i.e., nearly half the patients survive after a recurrence. However, death in the remaining half most frequently occurred at around three years post-recurrence, a much shorter period than the MST (four years). In contrast, patients with two metastases had a peak time of 3.2 years (for their hazard ratio), over one year longer than the MST (two years). Therefore, the combination of the five-year survival rate as assessed by the Kaplan–Meier curve and the peak time as assessed by HFA is closer to being the optimal prognostic indicator. HFA was also found to be quite useful in visualizing changes in death risk over time. Although HFA is widely used to analyze the occurrence of events not only in malignant diseases but also in benign diseases, such as surgical site infections [23, 24], the present study demonstrated its applicability to the survival analysis of recurrent colorectal cancer.
The present study has several limitations. First, the study cohort included old cases that lacked a sufficient pre- and post-recurrence surveillance period. Therefore, the survival outcome might have been better if the patients had received current, cytotoxic and molecularly targeted drugs. Second, this study was conducted at a single center, and a multicentric cohort study is needed to verify its findings.
In conclusion, we developed a novel method of assessing changes in mortality risk over time in patients with a postoperative recurrence of colorectal cancer using HFA. The findings of this study will hopefully be of use to physicians and patients alike in daily clinical practice.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Xi Y, Xu P (2021) Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol 14(10):101174. https://doi.org/10.1016/j.tranon.2021.101174
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K (2020) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25(1):1–42. https://doi.org/10.1007/s10147-019-01485-z
Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, Eguchi Nakajima T, Baba E, Tsuda M, Moriwaki T, Esaki T, Tsuji Y, Muro K, Taira K, Denda T, Funai S, Shinozaki K, Yamashita H, Sugimoto N, Okuno T, Nishina T, Umeki M, Kurimoto T, Takayama T, Tsuji A, Yoshida M, Hosokawa A, Shibata Y, Suyama K, Okabe M, Suzuki K, Seki N, Kawakami K, Sato M, Fujikawa K, Hirashima T, Shimura T, Taku K, Otsuji T, Tamura F, Shinozaki E, Nakashima K, Hara H, Tsushima T, Ando M, Morita S, Boku N, Hyodo I (2016) Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol 27(8):1539–1546. https://doi.org/10.1093/annonc/mdw206
Clark TG, Bradburn MJ, Love SB, Altman DG (2003) Survival analysis part I: basic concepts and first analyses. Br J Cancer 89(2):232–238. https://doi.org/10.1038/sj.bjc.6601118
Schober P, Vetter TR (2018) Survival analysis and interpretation of time-to-event data: the tortoise and the hare. Anesth Analg 127(3):792–798. https://doi.org/10.1213/ane.0000000000003653
Simes RJ, Zelen M (1985) Exploratory data analysis and the use of the hazard function for interpreting survival data: an investigator’s primer. J Clin Oncol 3(10):1418–1431. https://doi.org/10.1200/jco.1985.3.10.1418
James D, Mary K, Christian W (2017) TNM classification of malignant tumours, 8th edition
Kobayashi H, Mochizuki H, Sugihara K, Morita T, Kotake K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hase K, Oya M, Maeda K, Hirai T, Kameyama M, Shirouzu K, Muto T (2007) Characteristics of recurrence and surveillance tools after curative resection for colorectal cancer: a multicenter study. Surgery 141(1):67–75. https://doi.org/10.1016/j.surg.2006.07.020
Kawai K, Nozawa H, Hata K, Kiyomatsu T, Tanaka T, Nishikawa T, Sugihara K, Watanabe T (2018) Nomogram predicting survival after recurrence in patients with stage I to III colon cancer: a nationwide multicenter study. Dis Colon Rectum 61(9):1053–1062. https://doi.org/10.1097/dcr.0000000000001167
Nikolic N, Radosavljevic D, Gavrilovic D, Nikolic V, Stanic N, Spasic J, Cacev T, Castellvi-Bel S, Cavic M, Jankovic G (2021) Prognostic factors for post-recurrence survival in stage II and III colorectal carcinoma patients. Medicina (Kaunas) 57(10). https://doi.org/10.3390/medicina57101108
Doat S, Thiébaut A, Samson S, Ricordeau P, Guillemot D, Mitry E (2014) Elderly patients with colorectal cancer: treatment modalities and survival in France. National data from the ThInDiT cohort study. Eur J Cancer 50(7):1276–1283. https://doi.org/10.1016/j.ejca.2013.12.026
Lieu CH, Renfro LA, de Gramont A, Meyers JP, Maughan TS, Seymour MT, Saltz L, Goldberg RM, Sargent DJ, Eckhardt SG, Eng C (2014) Association of age with survival in patients with metastatic colorectal cancer: analysis from the ARCAD Clinical Trials Program. J Clin Oncol 32(27):2975–2984. https://doi.org/10.1200/jco.2013.54.9329
Lin BR, Chang TC, Lee YC, Lee PH, Chang KJ, Liang JT (2009) Pulmonary resection for colorectal cancer metastases: duration between cancer onset and lung metastasis as an important prognostic factor. Ann Surg Oncol 16(4):1026–1032. https://doi.org/10.1245/s10434-008-0286-3
Ryuk JP, Choi GS, Park JS, Kim HJ, Park SY, Yoon GS, Jun SH, Kwon YC (2014) Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann Surg Treat Res 86(3):143–151. https://doi.org/10.4174/astr.2014.86.3.143
Ueno H, Mochizuki H, Hatsuse K, Hase K, Yamamoto T (2000) Indicators for treatment strategies of colorectal liver metastases. Ann Surg 231(1):59–66. https://doi.org/10.1097/00000658-200001000-00009
O’Connell MJ, Campbell ME, Goldberg RM, Grothey A, Seitz JF, Benedetti JK, André T, Haller DG, Sargent DJ (2008) Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol 26(14):2336–2341. https://doi.org/10.1200/jco.2007.15.8261
Muto T, Kotake K, Koyama Y (2001) Colorectal cancer statistics in Japan: data from JSCCR registration, 1974–1993. Int J Clin Oncol 6(4):171–176. https://doi.org/10.1007/pl00012102
Kanazawa T, Watanabe T, Kazama S, Tada T, Koketsu S, Nagawa H (2002) Poorly differentiated adenocarcinoma and mucinous carcinoma of the colon and rectum show higher rates of loss of heterozygosity and loss of E-cadherin expression due to methylation of promoter region. Int J Cancer 102(3):225–229. https://doi.org/10.1002/ijc.10690
Nozoe T, Anai H, Nasu S, Sugimachi K (2000) Clinicopathological characteristics of mucinous carcinoma of the colon and rectum. J Surg Oncol 75(2):103–107. https://doi.org/10.1002/1096-9098(200010)75:2%3c103::aid-jso6%3e3.0.co;2-c
Catalano V, Loupakis F, Graziano F, Torresi U, Bisonni R, Mari D, Fornaro L, Baldelli AM, Giordani P, Rossi D, Alessandroni P, Giustini L, Silva RR, Falcone A, D’Emidio S, Fedeli SL (2009) Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer 100(6):881–887. https://doi.org/10.1038/sj.bjc.6604955
Negri FV, Wotherspoon A, Cunningham D, Norman AR, Chong G, Ross PJ (2005) Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol 16(8):1305–1310. https://doi.org/10.1093/annonc/mdi244
Kawai K, Ishihara S, Yamaguchi H, Sunami E, Kitayama J, Miyata H, Sugihara K, Watanabe T (2015) Nomograms for predicting the prognosis of stage IV colorectal cancer after curative resection: a multicenter retrospective study. Eur J Surg Oncol 41(4):457–465. https://doi.org/10.1016/j.ejso.2015.01.026
Arabía FA, Cantor RS, Koehl DA, Kasirajan V, Gregoric I, Moriguchi JD, Esmailian F, Ramzy D, Chung JS, Czer LS, Kobashigawa JA, Smith RG, Kirklin JK (2018) Interagency registry for mechanically assisted circulatory support report on the total artificial heart. J Heart Lung Transplant 37(11):1304–1312. https://doi.org/10.1016/j.healun.2018.04.004
Auerbach SR, Cantor RS, Bradford TT, Bock MJ, Skipper ER, Koehl DA, Butler K, Alejos JC, Edens RE, Kirklin JK (2022) The effect of infectious complications during ventricular assist device use on outcomes of pediatric heart transplantation. Asaio J 68(2):287–296. https://doi.org/10.1097/mat.0000000000001442
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
Ichiro Ise wrote the main manuscript, figures and tables. Kazushige Kawai, Daisuke Nakano, Misato Takao, Soichiro Natsume, Hiroki Kato, Sakiko Nakamori, Akira Dejima, Tatsuro Yamaguchi read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Synopsis for table of contents
Mean survival time is widely used as the indicator of prognosis in patients with a recurrence of colorectal cancer (CRC). As an alternative to MST, the present study established a novel method of visualizing the changes in mortality risk over time using hazard function analysis in patients with a postoperative CRC recurrence.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ise, I., Kawai, K., Nakano, D. et al. Hazard function analysis of prognosis after recurrent colorectal cancer. Langenbecks Arch Surg 409, 123 (2024). https://doi.org/10.1007/s00423-024-03308-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-024-03308-w