Abstract
Purpose
Postoperative complications after major liver surgery are common. Thoracic epidural anesthesia may provide beneficial effects on postoperative outcome. We strove to compare postoperative outcomes in major liver surgery patients with and without thoracic epidural anesthesia.
Methods
This was a retrospective cohort study in a single university medical center. Patients undergoing elective major liver surgery between April 2012 and December 2016 were eligible for inclusion. We divided patients into two groups according to whether or not they had thoracic epidural anesthesia for major liver surgery. The primary outcome was postoperative hospital length of stay, i.e., from day of surgery until hospital discharge. Secondary outcomes included 30-day postoperative mortality and major postoperative complications. Additionally, we investigated the effect of thoracic epidural anesthesia on perioperative analgesia doses and the safety of thoracic epidural anesthesia.
Results
Of 328 patients included in this study, 177 (54.3%) received thoracic epidural anesthesia. There were no clinically important differences in postoperative hospital length of stay (11.0 [7.00–17.0] vs. 9.00 [7.00–14.0] days, p = 0.316, primary outcome), death (0.0 vs. 2.7%, p = 0.995), or the incidence of postoperative renal failure (0.6 vs. 0.0%, p = 0.99), sepsis (0.0 vs. 1.3%, p = 0.21), or pulmonary embolism (0.6 vs. 1.4%, p = 0.59) between patients with or without thoracic epidural anesthesia. Perioperative analgesia doses — including the intraoperative sufentanil dose (0.228 [0.170–0.332] vs. 0.405 [0.315–0.565] μg·kg−1·h−1, p < 0.0001) — were lower in patients with thoracic epidural anesthesia. No major thoracic epidural anesthesia-associated infections or bleedings occurred.
Conclusion
This retrospective analysis suggests that thoracic epidural anesthesia does not reduce postoperative hospital length of stay in patients undergoing major liver surgery — but it may reduce perioperative analgesia doses. Thoracic epidural anesthesia was safe in this cohort of patients undergoing major liver surgery. These findings need to be confirmed in robust clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver resections are major surgeries with high rates of postoperative complications [1,2,3]. To reduce postoperative complications and enhance recovery after surgery, attempts to implement standardized interdisciplinary concepts have been made [4]. Whether thoracic epidural anesthesia should be routinely used in patients undergoing hepatic resection surgery remains controversial. On the one hand, thoracic epidural anesthesia potentially helps control pain and reduce intraoperative opioid doses [5]. Additionally, thoracic epidural anesthesia may mitigate systemic inflammation and improve coagulation, pulmonary function, and intestinal perfusion and motility [6, 7]. On the other hand, thoracic epidural anesthesia itself can cause complications. Especially in cirrhotic patients with impaired coagulation or in patients undergoing expanded liver resection, thoracic epidural anesthesia bears the risk of bleeding and epidural hematoma [8,9,10]. Only few studies investigated effects of thoracic epidural anesthesia on hospital length of stay and postoperative complications in patients undergoing liver surgery — and they revealed contrasting results [11,12,13,14,15].
In our institution, thoracic epidural anesthesia was routinely used for liver resection from January 2012 to June 2015, but was not routinely used thereafter. We aimed to investigate effects of thoracic epidural anesthesia on postoperative outcomes in patients undergoing major liver surgery. Specifically, we performed a retrospective cohort study to test the primary hypothesis that postoperative hospital length of stay is shorter in major liver surgery patients with than in patients without thoracic epidural anesthesia. Additionally, we investigated the effect of thoracic epidural anesthesia on 30-day postoperative mortality, major postoperative complications, and perioperative analgesia doses, and the safety of thoracic epidural anesthesia.
Material and methods
Ethics
The ethics committee approved the study and waived the need to obtain informed consent for collection, analysis, and publication of data for this retrospective cohort study (Name and address of ethics committee: Ethikkommission der Ärztekammer Hamburg, Weidestraße 122 b, 22083 Hamburg, Germany; President of ethics committee: Prof. Dr. med. M. Carstensen; Process No. WF-022/17; Date of approval: 04/26/2017).
Study design
This was a retrospective observational cohort study at the University Medical Center Hamburg-Eppendorf (Hamburg, Germany) to investigate the effect of thoracic epidural anesthesia on postoperative outcomes in patients undergoing major liver surgery.
Inclusion and exclusion criteria
Adult patients undergoing elective major liver surgery (hemi-hepatectomy, atypical liver resection, or biliodigestive anastomosis as well as bile duct revisions and surgical treatment of liver cysts) between April 1, 2012, and December 31, 2016, were eligible for study inclusion. We aimed to include approximately 150 patients per group. Exclusion criteria were laparoscopic liver surgery, explorative laparotomy, and redo procedures. Further, patients after liver transplantation or patients undergoing combined surgery for different organs were not included. In addition, we excluded patients if the anesthesia records or medical charts were incomplete.
Exposure
Patients were divided into two groups according to whether or not they had thoracic epidural anesthesia for major liver surgery. Thoracic epidural anesthesia was used per institutional routine since January 2012 (unless there were contraindications). In June 2015, anesthesiologists and surgeons agreed to stop routinely using thoracic epidural anesthesia in patients undergoing major liver surgery. This decision was based on reservations regarding the risk of bleeding associated with the use of thoracic epidural anesthesia in major liver surgery. In patients with thoracic epidural anesthesia, intraoperative thoracic epidural bolus injections of 0.25% bupivacaine with sufentanil 0.75 μg·ml−1 were used and repeated every 60–120 min, followed by postoperative continuous thoracic epidural anesthesia (6–8 ml·h−1 bupivacaine 0.125% with sufentanil 0.75 μg·ml−1 for patients < 70 years of age, 6–8 ml·h−1 bupivacaine 0.125% for patients ≥ 70 years of age). In addition, a patient-controlled bolus application of 2 ml was allowed every 15 min if needed.
Primary outcome
The primary outcome was postoperative hospital length of stay, i.e., from day of surgery until hospital discharge.
Secondary outcomes
Secondary outcomes were censored at postoperative day 30 and included postoperative complications (bile leakage, renal failure, sepsis, pulmonary arterial embolism, thrombosis of inferior cava vein, and pulmonary infiltrates or congestion on chest x-ray), intensive care unit length of stay, 30-day postoperative mortality, postoperative laboratory findings (liver and kidney values, coagulation profiles, and inflammation parameters), perioperative analgesia doses, and the safety of thoracic epidural anesthesia (occurrence of dura perforations, epidural infections, epidural abscess formation, epidural hematoma, or nerve injuries after epidural catheter placement or removal).
Data collection
Data were extracted from electronic health and anesthesia records (Soarian ® Clinicals, and Soarian ® Health Care Archive, Cerner Deutschland GmbH, Idstein, Germany).
Statistical analysis
Descriptive statistics are presented as median and interquartile range [IQR] for continuous variables and as total numbers and percentage for categorical variables. We compared baseline variables, preoperative laboratory findings, and perioperative variables, between patients with and without thoracic epidural anesthesia using Mann–Whitney U tests (continuous variables) and Fisher’s exact (categorical variables). Multivariate analyses were used to compare primary outcome, postoperative complications, and 30-day postoperative mortality between patients with and without thoracic epidural anesthesia. In these multivariate analyses (binary logistic regression, Cox regression, or general linear modelling, depending on whether the outcome variable was binary, time-to-event, or continuous), the primary independent variable thoracic epidural anesthesia was always adjusted for coronary artery disease, diabetes mellitus, and preoperative diuretics — because these covariates had shown significant relations to thoracic epidural anesthesia. Other covariates were only included in the multivariate analyses if they were statistically significant. For the categorial outcomes postoperative renal failure, pulmonary embolism, pulmonary congestion, bile leakage, and thrombosis of inferior cava vein, the number of events were too few to render multivariate analysis feasible, since the number of included covariates would have been as high or even higher than the number of outcome events. For these outcomes, group comparisons were done by Fisher’s exact tests. As baseline laboratory values differed substantially between patients with and without thoracic epidural anesthesia, we also included baseline laboratory values as covariates for multivariate comparison of postoperative laboratory findings. For creatinine, international normalized ratio of prothrombin time (INR), and partial thromboplastin time (PTT), we also found significant effects for coronary artery disease which was included as covariable as well. The p values of two-tailed tests are presented. Since the nature of this study is explorative, no a priori sample size calculation was performed and no α error adjustments for multiple comparisons were made and two-tailed p values < 0.05 were considered statistically significant. All analyses were performed using SPSS v. 25 (IBM © Inc., Armonk, NY, USA).
Results
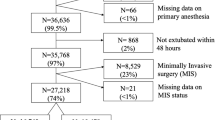
We screened 368 patients who met the inclusion criteria, but excluded 42 because they had redo surgery. For patients with thoracic epidural anesthesia, a time period between April 2012 and January 2014 was sufficient, and for patients without epidural anesthesia, a time period between April 2012 and February 2016 was needed to include a sufficient number of patients.
We thus analyzed data of 326 patients. One hundred seventy-seven of these 326 patients (54.3%) had thoracic epidural anesthesia (Table 1, Table 2). Patients without thoracic epidural anesthesia more often had coronary artery disease and diabetes mellitus and more often were treated with diuretics before surgery than patients with thoracic epidural anesthesia.
There was no clinically important difference in postoperative hospital length of stay between patients with and without thoracic epidural anesthesia (11.0 [7.00–17.0] vs. 9.00 [7.00–14.0] days, p = 0.316).
None of the 177 patients with thoracic epidural anesthesia but 4 of 149 patients without thoracic epidural anesthesia died within 30 days of surgery (p = 0.995). There were no clinical important differences in postoperative complications between patients with and without thoracic epidural anesthesia (Table 3).
Patients with thoracic epidural anesthesia had lower total doses of sufentanil compared to patients without thoracic epidural anesthesia (0.228 [0.170–0.332] vs. 0.405 [0.315–0.565] μg·kg−1·h−1, p < 0.0001), a difference that is clinically important. Moreover, piritramide doses as well as the proportions of patients who received metamizole, as well as clonidine, were lower among patients with than without thoracic epidural anesthesia (Table 4). Patients with thoracic epidural anesthesia received higher doses of colloids (2.23 [0.000–4.59] vs. 1.24 [0.000–3.65] ml·kg−1·h−1, p = 0.025).
In patients with thoracic epidural anesthesia, anesthesia induction took 15 min longer than in patients without thoracic epidural anesthesia (55.0 [5.0–60.0] vs. 40.0 [30.0–50.0] min, p < 0.0001), a difference that might be of clinical and economic importance. There were no clinical important differences in laboratory findings between patients with and without thoracic epidural anesthesia, except for C-reactive protein levels, which were higher among patients with than in patients without thoracic epidural anesthesia (Table 5).
Dura perforations occurred in 1.7% of patients with thoracic epidural anesthesia. The rate of intraoperative thoracic epidural bolus injection was 9.68 [7.81–11.5] ml·h−1. The duration of postoperative thoracic epidural anesthesia use was 5.0 [3.0–6.0] days. No patients had epidural infections, epidural abscess formation, epidural hematoma, or nerve injuries after epidural catheter placement or removal.
Discussion
In this retrospective cohort study, we revealed that thoracic epidural anesthesia does not reduce hospital length of stay in patients undergoing major liver surgery — but it may reduce required analgetic doses.
Thoracic epidural anesthesia may provide beneficial effects on postoperative outcome in patients undergoing major abdominal surgery [16]. In liver surgery, beneficial effects must be weighed against the increased risk of bleeding following liver resection, which may lead to increased complication rates associated with thoracic epidural anesthesia. Balance between beneficial effects and risks are scarcely investigated. Whether beneficial effects of thoracic epidural anesthesia in liver surgery translate into shorter hospital lengths of stay and less postoperative complications remains unknown. In our retrospective analysis, postoperative hospital length of stay did not differ between patients with and without thoracic epidural anesthesia. Moreover, there were no differences in intensive care unit length of stay between patients with and without thoracic epidural anesthesia. In line with our findings, a retrospective comparison of epidural anesthesia with intravenous patient-controlled analgesia in 226 patients for live liver donation also did not show a difference in hospital length of stay [17]. In contrast to our results, a retrospective analysis of 126 cirrhotic patients undergoing liver surgery in combination with a fast-track protocol demonstrated a shorter hospital length of stay in patients with epidural anesthesia than in patients without epidural anesthesia [11]. On the contrary, a retrospective matched cohort study revealed a longer hospital length of stay for patients receiving epidural anesthesia for liver surgery while a retrospective analysis of > 20,000 patients undergoing hepatopancreatic surgery also showed an increase in hospital length of stay in patients with epidural anesthesia. However, it should be mentioned that the proportion of patients having epidural anesthesia was considerably low in both studies [2, 12].
With regard to postoperative complications, in our study, rates of pulmonary or renal complications, bile leakage, and sepsis were similar between patients with and without thoracic epidural anesthesia. However, the complication rates were low and the number of patients thus may be insufficient to definitely explore the effect of thoracic epidural anesthesia on postoperative complications. Nevertheless, in line with our results, a retrospective analysis of 177 liver surgery patients found no differences in postoperative complications — although complication rates were considerably higher than in our study [18]. In a retrospective study comparing epidural anesthesia with patient-controlled analgesia following hepatectomy, overall postoperative outcomes were also similar between groups [19]. In contrast, a retrospective analysis in 829 patients having liver resections with and without epidural anesthesia revealed a potential risk for postoperative acute kidney injury associated with epidural anesthesia [20]. A recent propensity score-matched analysis showed no differences in hospital length of stay and postoperative complications between patients having liver surgery with and without epidural anesthesia — but an improvement in 1-year survival for patients receiving epidural anesthesia [14].
Epidural anesthesia provides beneficial effects on perioperative analgesia [2, 13, 17, 21]. It has been shown to provide effective postoperative analgesia in liver surgery [5, 19, 22]. In our study, intraoperative sufentanil doses were lower for patients with compared to patients without thoracic epidural anesthesia. Moreover, there were lower proportions of patients receiving non-opioid analgesics (metamizole) or co-analgesics (clonidine) in patients with compared to patients without epidural anesthesia. In this context, the increased duration of anesthesia duration attributed to epidural anesthesia would be inside an acceptable range for most physicians.
Sympathetic block induced by thoracic epidural anesthesia may lead to hypotension requiring administration of fluids or vasopressors. Higher rates of hypotension and higher amounts of administered fluids as well as vasopressors in patients with epidural anesthesia were previously reported [18, 23]. This may be in line with our study showing a higher amount of colloids in patients with compared to patients without thoracic epidural anesthesia. However, the amount of crystalloids was not different between patents with compared to patients without thoracic epidural anesthesia and we did not find clinical important differences of vasopressor doses. Furthermore, we could not find a higher proportion of renal failure, pulmonary congestion, or other complications in patients with thoracic epidural anesthesia that could be attributed to hypotension or negative effects of potentially increased fluid requirements.
Sympatholytic effects of thoracic epidural anesthesia may lead to improvements of intestinal motility. We retrospectively analyzed occurrence of defecation prior the second postoperative day and could not find advantages for patients with thoracic epidural anesthesia. In line with our study, no advantages of epidural anesthesia after liver surgery in regard to vomiting or first fluid intake were reported [17]. In contrast, a Cochrane meta-analysis revealed a high quality of evidence for acceleration of gastrointestinal transit after major surgery when epidural anesthesia was used [24]. Beneficial effects of thoracic epidural anesthesia may have possibly been found for our patient collective undergoing major liver surgery by use of different parameters of gastrointestinal function.
A major concern when using epidural anesthesia in liver surgery is increased risks of bleeding due to a changed postoperative coagulation function [25]. Reductions in coagulation prior liver surgery may preclude the use of epidural anesthesia. In our patient collective, neuraxial anesthesia was performed in accordance with the actual guidelines precluding patients with impaired coagulation or existing use of anticoagulants or antiplatelet drugs within certain time intervals prior surgery. Moreover, changes in coagulation profile including thrombocytopenia are frequently observed after liver resection due to perioperative blood loss or impairments of liver function [8, 26, 27]. Concerns about the risk of epidural hematoma or prolonged duration of indwelling catheters caused by coagulation derangements have been raised in this regard. Safety issues for use of epidural anesthesia in liver surgery have been proposed. However, safe use of epidural anesthesia has been shown despite coagulation changes after liver resection [5, 9, 25, 26, 28]. Although some authors reported delayed withdrawal of epidural catheters caused by coagulation derangements, this has not led to the formation of epidural hematoma or abscess [8, 18]. In our study, there were no important changes of coagulation profile in the postoperative period between patients with and patients without thoracic epidural anesthesia, although thrombocytes were reduced in both groups. Moreover, there was no incidence of epidural hematoma, epidural abscess, or nerve injuries. Therefore, our study supports the safe use of epidural anesthesia in major liver surgery. Nevertheless, given the very low overall incidence of epidural hematoma [29, 30], robust prospective studies are needed to adequately address this issue.
Epidural anesthesia itself can affect coagulation. Reduction of thrombotic events has been shown for epidural anesthesia leading to improvements of outcome. While the mechanisms are not fully understood, they include influences on coagulation, fibrinolysis, inflammation, and platelet aggregation [7]. As mentioned, there were no clinical important differences in thrombocytes and coagulation profile between patients with and without thoracic epidural anesthesia. Moreover, in regard to thrombotic risks, there were no differences in the occurrence of inferior vena cava thrombosis or pulmonary embolism between patients with and patients without thoracic epidural anesthesia.
In our retrospective analysis, four patients died within 30 days. Causes of death were respiratory failure in one case, sepsis in two cases, and thrombosis of hepatic vein in one case. Although all of them were in the group of patients without epidural anesthesia, multivariate analysis did not reveal a significant effect between groups. In addition, this was a retrospective study and mortality was included only as secondary outcome variable. Prospective studies with a higher patient number are needed to address potential beneficial effects of thoracic epidural anesthesia on postoperative mortality after major liver surgery in the future.
Recently, a number of studies have compared effects of epidural anesthesia with local wound infiltration techniques in patients undergoing major liver surgery [23, 31, 32]. In our study, no patient received local anesthesia techniques and the comparison between local and epidural anesthesia is beyond the scope of this study. While there has been a huge number of studies addressing the effect of epidural anesthesia on hospital length of stay and postoperative complications in major surgery [24, 33, 34], our analysis was restricted to patients having major liver surgery and therefore is not generalizable to other types of surgery — specifically thoracic and gastrointestinal surgery. However, we deliberately focused on liver surgery to gather insights on the balance between potential benefits and the specific risks associated with epidural anesthesia for this patient collective. As further limitation, it should be considered that apart from analgesia use in the perioperative period, we did not assess additional parameters of analgesia including total postoperative analgesia consumption, subjective values like numeric rating scale scores, postoperative nausea, or endocrine parameters. In addition, rates of complication were generally low for both groups possibly reducing the ability to show differences of outcome parameters for this patient collective. In our institution, thoracic epidural anesthesia was routinely used for liver resection from January 2012 to June 2015, but was not used thereafter. This decision was based on reservations about potential bleeding risks that may be associated with the use of thoracic epidural anesthesia in major liver surgery. Thus, indication for thoracic epidural anesthesia was only secondary based on comorbidities or surgical extent minimizing selection bias. The chosen time interval was intended to reduce selection bias and most baseline values were comparable between groups. Nonetheless, concomitant diseases were not comparable for all parameters between groups, thus representing potential sources of confounding. Therefore, as described in the statistics section, we performed multivariate analysis and included coronary artery disease, diabetes mellitus, and application of diuretics as covariables because they had shown significant associations with use of thoracic epidural anesthesia (data not shown). However, for the categorial outcome variables perioperative renal failure, pulmonary embolism, pulmonary congestion, bile leakage, and thrombosis of inferior cava vein, the number of events were too few to render multivariate analysis feasible as described in the statistics section. Although this may have reduced potential bias, our results should be confirmed in robust prospective trials.
Conclusion
In conclusion, this retrospective analysis suggests that thoracic epidural anesthesia does not reduce hospital length of stay in patients undergoing major liver surgery — but it may reduce perioperative analgesia doses. Thoracic epidural anesthesia did not lead to important differences in postoperative complications and was safe in this cohort of patients undergoing major liver surgery. These findings need to be confirmed in robust clinical trials.
Data availability
Original data will be provided upon reasonable request.
Abbreviations
- ACE:
-
angiotensin converting enzyme
- AST:
-
aspartate transaminase
- AT1-receptor:
-
angiotensin-II receptor type 1
- CRP:
-
C-reactive protein
- GGT:
-
gamma-glutamyl-transferase
- INR:
-
international normalized ratio of prothrombin time
- PACU:
-
post-anesthesia care unit
- PTT:
-
partial thromboplastin time
References
Hughes MJ, Ventham NT, Harrison EM, Wigmore SJ (2015) Central venous pressure and liver resection: a systematic review and meta-analysis. HPB (Oxford) 17(10):863–871
Rosero EB, Cheng GS, Khatri KP, Joshi GP (2014) Evaluation of epidural analgesia for open major liver resection surgery from a US inpatient sample. Proc (Bayl Univ Med Cent) 27(4):305–312
Jones C, Kelliher L, Dickinson M, Riga A, Worthington T, Scott MJ et al (2013) Randomized clinical trial on enhanced recovery versus standard care following open liver resection. Br J Surg 100(8):1015–1024
Melloul E, Hubner M, Scott M, Snowden C, Prentis J, Dejong CH et al (2016) Guidelines for perioperative care for liver surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg 40(10):2425–2440
Ganapathi S, Roberts G, Mogford S, Bahlmann B, Ateleanu B, Kumar N (2015) Epidural analgesia provides effective pain relief in patients undergoing open liver surgery. Br J Pain 9(2):78–85
Freise H, Van Aken HK (2011) Risks and benefits of thoracic epidural anaesthesia. Br J Anaesth 107(6):859–868
Hahnenkamp K, Theilmeier G, Van Aken HK, Hoenemann CW (2002) The effects of local anesthetics on perioperative coagulation, inflammation, and microcirculation. Anesth Analg 94(6):1441–1447
Takita K, Uchida Y, Hase T, Kamiyama T, Morimoto Y (2014) Co-existing liver disease increases the risk of postoperative thrombocytopenia in patients undergoing hepatic resection: implications for the risk of epidural hematoma associated with the removal of an epidural catheter. J Anesth 28(4):554–558
Stamenkovic DM, Jankovic ZB, Toogood GJ, Lodge JP, Bellamy MC (2011) Epidural analgesia and liver resection: postoperative coagulation disorders and epidural catheter removal. Minerva Anestesiol 77(7):671–679
Tzimas P, Prout J, Papadopoulos G, Mallett SV (2013) Epidural anaesthesia and analgesia for liver resection. Anaesthesia 68(6):628–635
Siniscalchi A, Gamberini L, Bardi T, Laici C, Gamberini E, Francorsi L et al (2016) Role of epidural anesthesia in a fast track liver resection protocol for cirrhotic patients — results after three years of practice. World J Hepatol 8(26):1097–1104
Merath K, Hyer JM, Mehta R, Bagante F, Paredes A, Wu L et al (2019) Use of perioperative epidural analgesia among Medicare patients undergoing hepatic and pancreatic surgery. HPB (Oxford) 21(8):1064–1071
Hughes M, McNally S, McKeown DW, Wigmore S (2015) Effect of analgesic modality on outcome following open liver surgery: a systematic review of postoperative analgesia. Minerva Anestesiol 81(5):541–556
Knaak C, Spies C, Schneider A, Jara M, Vorderwulbecke G, Kuhlmann AD et al (2020) Epidural anesthesia in liver surgery—a propensity score-matched analysis. Pain Med 21(11):2650–2660
Su Y, Pu Y, Zhao Z, Yang X (2020) Influence of combined epidural anesthesia on cognitive function, inflammation and stress response in elderly liver cancer patients undergoing surgery. Oncol Lett 19(4):2733–2738
Miller RD (2015) Miller’s anesthesia, 8th edn. Elsevier, Philadelphia
Clarke H, Chandy T, Srinivas C, Ladak S, Okubo N, Mitsakakis N et al (2011) Epidural analgesia provides better pain management after live liver donation: a retrospective study. Liver Transpl 17(3):315–323
Revie EJ, Massie LJ, McNally SJ, McKeown DW, Garden OJ, Wigmore SJ (2011) Effectiveness of epidural analgesia following open liver resection. HPB (Oxford) 13(3):206–211
Allen S, DeRoche A, Adams L, Slocum KV, Clark CJ, Fino NF et al (2017) Effect of epidural compared to patient-controlled intravenous analgesia on outcomes for patients undergoing liver resection for neoplastic disease. J Surg Oncol 115(4):402–406
Kambakamba P, Slankamenac K, Tschuor C, Kron P, Wirsching A, Maurer K et al (2015) Epidural analgesia and perioperative kidney function after major liver resection. Br J Surg 102(7):805–812
Schreiber KL, Chelly JE, Lang RS, Abuelkasem E, Geller DA, Marsh JW et al (2016) Epidural versus paravertebral nerve block for postoperative analgesia in patients undergoing open liver resection: a randomized clinical trial. Reg Anesth Pain Med 41(4):460–468
Hausken J, Fretland AA, Edwin B, Andersen MH, Dagenborg VJ, Bjornelv GMW et al (2019) Intravenous patient-controlled analgesia versus thoracic epidural analgesia after open liver surgery: a prospective, randomized, controlled, noninferiority trial. Ann Surg 270(2):193–199
Bell R, Ward D, Jeffery J, Toogood GJ, Lodge JA, Rao K et al (2019) A randomized controlled trial comparing epidural analgesia versus continuous local anesthetic infiltration via abdominal wound catheter in open liver resection. Ann Surg 269(3):413–419
Guay J, Nishimori M, Kopp SL (2016) Epidural local anesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting, and pain after abdominal surgery: a Cochrane review. Anesth Analg 123(6):1591–1602
Elterman KG, Xiong Z (2015) Coagulation profile changes and safety of epidural analgesia after hepatectomy: a retrospective study. J Anesth 29(3):367–372
Choi SJ, Gwak MS, Ko JS, Kim GS, Ahn HJ, Yang M et al (2007) The changes in coagulation profile and epidural catheter safety for living liver donors: a report on 6 years of our experience. Liver Transpl 13(1):62–70
Karna ST, Pandey CK, Sharma S, Singh A, Tandon M, Pandey VK (2015) Postoperative coagulopathy after live related donor hepatectomy: incidence, predictors and implications for safety of thoracic epidural catheter. J Postgrad Med 61(3):176–180
Koul A, Pant D, Rudravaram S, Sood J (2018) Thoracic epidural analgesia in donor hepatectomy: an analysis. Liver Transpl 24(2):214–221
Volk T, Wolf A, Van Aken H, Burkle H, Wiebalck A, Steinfeldt T (2012) Incidence of spinal haematoma after epidural puncture: analysis from the German network for safety in regional anaesthesia. Eur J Anaesthesiol 29(4):170–176
Cook TM, Counsell D, Wildsmith JA, Royal College of Anaesthetists Third National Audit Project (2009) Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth 102(2):179–190
Bell R, Pandanaboyana S, Prasad KR (2015) Epidural versus local anaesthetic infiltration via wound catheters in open liver resection: a meta-analysis. ANZ J Surg 85(1-2):16–21
Gavriilidis P, Roberts KJ, Sutcliffe RP (2019) Local anaesthetic infiltration via wound catheter versus epidural analgesia in open hepatectomy: a systematic review and meta-analysis of randomised controlled trials. HPB (Oxford) 21(8):945–952
Cummings KC III, Zimmerman NM, Maheshwari K, Cooper GS, Cummings LC (2018) Epidural compared with non-epidural analgesia and cardiopulmonary complications after colectomy: a retrospective cohort study of 20,880 patients using a national quality database. J Clin Anesth 47:12–18
Kampe S, Weinreich G, Darr C, Eicker K, Stamatis G, Hachenberg T (2014) The impact of epidural analgesia compared to systemic opioid-based analgesia with regard to length of hospital stay and recovery of bowel function: retrospective evaluation of 1555 patients undergoing thoracotomy. J Cardiothorac Surg 9:175
Acknowledgements
We would like to thank Prof. Bernd Saugel (Department of Anesthesiology, Center of Anesthesiology and Intensive Care Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany) for his assistance with manuscript drafting.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
CRB, GG, MFG, SF, RN, CJCT, and SAH designed the study. CRB and SAH wrote the manuscript. CRB, JCW, CJCT, and SAH collected the data. CRB, JCW, HOP, CJCT, and SAH interpreted the data. HOP performed the statistical analysis. CJCT and SAH supervised the study. All authors critically revised the manuscript for important intellectual content. All authors approved the final manuscript. All authors agree to be accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics committee approved the study and waived the need to obtain informed consent for collection, analysis, and publication of data for this retrospective cohort study (Name and address of ethics committee: Ethikkommission der Ärztekammer Hamburg, Weidestraße 122 b, 22083 Hamburg, Germany; President of ethics committee: Prof. Dr. med. M. Carstensen; Process No. WF-022/17; Date of approval: 04/26/2017).
Competing interests
Constantin J. C. Trepte has received honorary for lectures by Maquet. All other authors declare no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary file 1
Strobe Statement—Checklist of items that should be included in reports of cohort studies
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Behem, C.R., Wegner, J.C., Pinnschmidt, H.O. et al. Effect of thoracic epidural anesthesia on postoperative outcome in major liver surgery: a retrospective cohort study. Langenbecks Arch Surg 408, 168 (2023). https://doi.org/10.1007/s00423-023-02900-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-02900-w