Abstract
Purpose
Pylorus-preserving pancreatoduodenectomy (PPPD) has been the gold standard for pancreatic head lesion resection for several years. Some studies have noted that it involves more delayed gastric emptying (DGE) than classical Whipple (i.e., pancreatoduodenectomy with antrectomy). Our working hypothesis was that the classical Whipple has a lower incidence of DGE. We aimed to compare the incidence of DGE among pancreatoduodenectomy techniques.
Methods
This pragmatic, randomized, open-label, single-center clinical trial involved patients who underwent classical Whipple (study group) or PPPD (control group). Gastric emptying was clinically evaluated using scintigraphy. DGE was defined according to the International Study Group of Pancreatic Surgery (ISGPS) criteria. The secondary endpoints were postoperative morbidity, length of hospital stay, anthropometric measurements, and nutritional status.
Results
A total of 84 patients were randomized (42 per group). DGE incidence was 50% (20/40, 95% confidence interval (95% CI): 35–65%) in the study group and 62% (24/39, 95% CI: 46–75%) in the control group (p = 0.260). No differences were observed between both groups regarding postoperative morbidity or length of hospital stay. Anthropometric measurements at 6 months post-surgery: triceps fold measurements were 12 mm and 16 mm (p = 0.021). At 5 weeks post-surgery, triceps fold measurements were 13 mm and 16 mm (p = 0.020) and upper arm circumferences were 26 cm and 28 cm (p = 0.030). No significant differences were observed in nutritional status.
Conclusion
DGE incidence and severity did not differ between classical Whipple and PPPD. Some anthropometric measurements may indicate a better recovery with PPPD.
Trial registration
ClinicalTrials.gov Identifier: NCT03984734.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatoduodenectomy is the gold standard technique for treating the tumors of the periampullary area [1, 2]. Although the initial description (classical Whipple) included antrectomy, pyloric preservation has been successful by digestive surgeons in recent years. Advocates argue that it achieves lower blood loss and better quality of life (QoL) [3, 4]. However, subsequent studies showed that pyloric preservation might be associated with an increase in delayed gastric emptying (DGE) [5,6,7,8,9]. Conversely, meta-analyses [10,11,12] showed no differences between the incidence of DGE in pylorus-preserving pancreatoduodenectomy (PPPD) and classical Whipple. This issue is controversial and a matter of concern [13].
According to the International Study Group of Pancreatic Surgery (ISGPS) guidelines on nutritional support and therapy in pancreatic surgery, patients who undergo pancreatoduodenectomy should be carefully monitored to assess the presence of endocrine and/or exocrine pancreatic insufficiency and nutritional status [14]. However, to our knowledge, no data are available on anthropometric changes after pancreatoduodenectomy.
Our working hypothesis was that the classical Whipple has a lower DGE incidence. Therefore, we aimed to compare the incidence of DGE between pancreatoduodenectomies (classical Whipple or pylorus preservation) after surgery in this randomized clinical trial (RCT). Similarly, we aimed to provide comparative data on postoperative morbidity, nutritional status, and anthropometric measurements after pancreatoduodenectomy.
Materials and methods
Study design
This was a randomized (1:1), open-label, single-center, controlled, parallel-group, pragmatic clinical trial of patients who underwent pancreatoduodenectomy.
The study protocol was approved by the Ethics Committee (185/03) of the Bellvitge University Hospital, University of Barcelona, and it was registered at ClinicalTrials.gov (QUANUPAD Trial; NCT03984734). Written informed consent was obtained from all the patients. The study was conducted in accordance with the updated Declaration of Helsinki, guidelines for Good Clinical Practice, and applicable Spanish regulatory requirements. Confidentiality was guaranteed in accordance with current Spanish legislation (LOPD 15/1999—currently repealed, LOPD 3/2018). This manuscript was written in accordance with the CONSORT guidelines [15].
Study population
This study included adult patients (≥ 18 years of age) of both sexes who underwent surgical partial pancreatoduodenectomy (pancreatic head resection) at the Bellvitge University Hospital and provided written informed consent. Exclusion criteria were as follows: (i) patients who underwent total pancreatectomy; (ii) patients who underwent incomplete pancreatoduodenectomy; (iii) patients who received associated resections of other organs, except for the portal or superior mesenteric veins; (iv) patients with previous gastrectomy or other gastric surgery; (v) patients receiving neoadjuvant treatment; (vi) patients with liver cirrhosis; and (vii) patients with duodenal ischemia or tumor infiltration that required an antrectomy.
The following anonymized data were entered into an ad hoc-created case report form (CRF): date of birth, date of diagnosis, date of surgery, anthropometric measurements (weight, upper arm circumference, tricipital skinfold), scintigraphy study of gastric emptying, nutritional status (analytical variables: liver enzymes, albumin, prealbumin, C-reactive protein (CRP)), readmissions, morbidity, and QoL.
Randomization and masking
An external statistical consultant created a randomization list (1:1) before the start of the study. Patients were allocated to one of two study groups: (1) study group: patients who underwent classical Whipple, or (2) control group: patients who underwent PPPD.
The external statistician’s team, the only one with access to the randomization list, prepared envelopes (one per patient) containing the randomization code. They were opaque, sealed, and numbered sequentially. Randomization was performed by opening the envelope with a blinded assistant of the surgical team.
Randomization was performed before starting resection, as described in another study [16]. The randomization timing (before starting the resection) was selected to avoid including patients with exclusion criteria only detectable at the time of the surgery, such as (i) an incomplete pancreatoduodenectomy due to intraoperative findings (i.e., liver metastasis) and (ii) other intraoperative findings that require a change of course in the previously programmed surgical plan (e.g., total pancreatectomy due to an affected margin).
This was an open-label study in which both the patient and the surgeon knew which type of reconstruction was conducted. A blinded third-party evaluation of the primary endpoint was not performed.
Study procedure
The study duration was from the surgery day (day 0, randomization) to 6 months after surgery. The patient was monitored daily from the surgery day until the day of hospital discharge. Outpatient control visits were scheduled for the first week after discharge and the 5th week and 6th month after surgery (day 0).
Surgical technique
All interventions were performed by a team of surgeons with experience in hepatobiliary and pancreatic surgeries. All surgeons specialized in hepatobiliary surgery and liver transplantation.
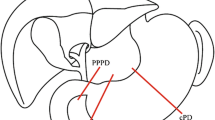
All patients underwent resection with curative intent, including partial pancreatoduodenectomy with standard lymphadenectomy [17, 18]. We started the surgery with exploratory laparoscopy to rule out previously undetected disease extensions. A right subcostal laparotomy was performed, followed by cholecystectomy with lymphadenectomy of the hepatic hilum. Duodenal or gastric transections were obtained using a stapler. Resection surgery was performed according to international cancer standards. In the study group, patients underwent classical Whipple, and distal gastrectomy varying from 20 to 40% was performed. In the control group, patients underwent PPPD; in these cases, the right gastric artery was preserved unless the artery restricted gastric mobility. The duodenum was dissected and divided at least 2 cm distally to the pylorus. Reconstruction was performed on a single loop, starting with pancreatic anastomosis. Pancreatic, biliary, and gastric anastomoses were performed in a retrocolic position. Duct-to-mucosa pancreatojejunostomy was the first choice for all patients. In the case of a narrow pancreatic duct, an internal transanastomotic pancreatic duct stent was introduced after pancreatojejunostomy at the surgeon’s discretion. Hepaticojejunostomy was performed approximately 15 cm from pancreatojejunostomy.
Gastric or duodenal anastomosis was performed depending on the randomization group. Retrocolic duodenoenteric or gastroenteric anastomoses were performed using silk sutures. In cases of duodenal ischemia at the time of suturing or massive periampullary tumors with possible duodenal affectation, the duodenum was divided, and a gastroenteric anastomosis was performed. These patients were excluded from the final data analysis. These anastomoses were performed approximately 60 cm from the biliary anastomosis site. Braun enteroenterostomies were not performed. In both groups, two drains were placed close to the pancreatojejunal anastomosis (n = 2) and one posteriorly to the hepaticojejunal anastomosis (Bellovac, Wellspect, HealthCare, Möndal, Sweden). After surgery, a nasogastric suction tube was placed in all patients.
Postoperative care
The analgesic treatment protocol and diet progression scheme during the postoperative period were identical between the two groups. During the first 24 h, the patients were monitored in an intensive care unit under the care of the anesthesia department. All patients were administered opioid- and non-steroidal anti-inflammatory drug-based analgesics, antiemetics every 8 h, and nasogastric aspiration. After 24 h, patients were transferred to the surgical ward, and analgesics were administered at the surgeon’s discretion. The nasogastric tube was maintained until the suction debit was less than 800 mL/day, and correct clamping tolerance was observed (i.e., with no clinical or radiological suspicion of gastric stasis). In cases of clinical doubt, a gastrointestinal transit study with oral contrast was performed, and the nasogastric tube was removed when there was evidence of contrast passage across the anastomosis. Patients were provided total parenteral nutrition if no oral tolerance was achieved by the 7th day. Metoclopramide and ondansetron were administered every 6 h on an alternating regimen to all patients until the nasogastric tube was removed. In cases of fever or suspected sepsis, an abdominal CT scan was performed to rule out an intra-abdominal abscess. After removal of the nasogastric tube for at least 7 days, treatment with antiemetics was maintained until progressive withdrawal at the surgeon’s discretion. Amylase levels in the drainage fluid were measured on the first and third days after surgery. The drains were removed when amylase levels were less than threefold those in the blood. None of the patients received erythromycin or somatostatin [19, 20].
Follow-up
Before surgery (day 0), baseline characteristics, anthropometric measurements, nutritional status (laboratory variables—see the “Outcomes” section for more detail), scintigraphy study of gastric emptying, and QoL questionnaires were gathered. Study visits were scheduled during the perioperative period. Clinical follow-up of all patients was performed by the same team of surgeons. All patients visited the hospital twice during the first 90 days: the first week after discharge and the 5th week after surgery. The follow-up was performed during outpatient visits by the surgeon in charge. In the 5th-week control visit after surgery, anthropometric measurements and nutritional status (laboratory variables) were recorded, and a scintigraphy gastric emptying study was performed. Data on readmission and morbidity at 90 days after surgery were obtained from the medical files. During the control visit at 6 months, nutritional status (laboratory variables) and anthropometric measurements were reobtained, and patients were required to answer a new QoL questionnaire.
Outcomes
The primary efficacy endpoints were the incidence and severity of DGE. Gastric emptying was clinically evaluated using scintigraphy. DGE and its severity (DGE grade) were defined according to the ISGPS criteria [3, 21].
Scintigraphy of gastric emptying was performed according to the hospital’s protocol. In all patients, gastric emptying at orthostasis was measured before surgery and at 5 weeks post-surgery. The patients were radiolabeled with 1 mCi (37 Mbq) of 99mTc-colloid mixed with eggs, two slices of bread, and 200 mL of water (ingestion time < 10 min). Following ingestion, gastric area images were obtained at 15-min intervals for 90 min using a single-head gamma camera equipped with a 140-keV high-resolution collimator. The gastric area and background regions of interest were outlined in the first image and projected on the following images, and the percentage of retained gastric activity (GR) at 90 min versus the baseline image was calculated [22]. Pathological gastric emptying (DGE) was defined as gastric retention according to scintigraphic criteria (isotope that remains in the stomach) in patients with a radiotracer percentage greater than 61% at 90 min [22].
The secondary endpoints were safety (postoperative morbidity), length of hospital stay, anthropometric measurements, nutritional status, and QoL.
Postoperative morbidity was defined according to the Clavien–Dindo classification [23]. Postoperative morbidity encompassed the appearance of any kind of morbidity during the hospital stay, and the final decision regarding its presence was made by consensus among the surgical team members after the daily visit. Perioperative mortality was defined as death during the same hospital admission and within 90 days after surgery if the patient was discharged early.
Information on whether the patient had a narrow pancreatic duct and/or a pancreatic fistula was also gathered. A narrow pancreatic duct was defined as a diameter ≤ 3 mm. A pancreatic fistula was defined as the presence of an outflow of amylase-rich drainage fluid after the 3rd postoperative day and was classified according to the International Study Group for Pancreatic Fistula criteria [24].
Readmissions during the first 90 days after the surgery were recorded. Patients with postoperative death were excluded from the DGE analysis.
The anthropometric measurements used were weight, arm circumference, and triceps skinfolds. These were recorded in the preoperative period and at 5 and 6 months after surgery [25]. The upper arm circumference, assessed at the midpoint of the proximal arm (cm), was measured using a measuring tape. The tricipital skinfold (mm) was measured using a plicometer and a dermatograph pencil. In accordance with the Bistrian and Blackburn criteria [26], the average upper arm circumference values accepted were 24.3 cm in men and 17.7 cm in women. Regarding the tricipital skinfold, the values accepted were 12.5 mm in men and 22.3 mm in women.
Nutritional status was assessed using the following laboratory tests: liver enzymes, albumin, prealbumin, creatinine, urea, and CRP, at the preoperative visit and at the 5 weeks and 6 months post-surgery visits. All laboratory tests were performed according to the quality standards of the reference laboratory.
QoL was evaluated using the QLQ-PAN26 questionnaire [27], which explores different areas and assigns a value to each answer. A numerical result was obtained, and the higher the value, the worse the QoL. QoL was evaluated at the preoperative visit and at the 6-month postoperative visit.
Statistical analysis
The sample size was calculated based on the incidence of DGE after pancreatoduodenectomy. The expected incidence of DGE after PPPD (control group) was 43% [5], and that after classical Whipple (study group) was 10% [5]. To detect differences in the contrast of the null hypothesis (H0: p1 = p2) using a two-sided χ2 test for two independent samples, with an α error of 0.05, a statistical power of 0.90, and a dropout rate of 10%, 40 patients were required in each group (1:1).
The data were encrypted and stored in a database created using Microsoft Access® (Microsoft, Redmond, WA, USA). The statistical analysis was only based on the “full analysis” set. Continuous variables are reported as mean (standard deviation (SD)). Variables that followed a normal distribution were analyzed using Student’s t-test for the comparison of means and the χ2 test (Fisher’s exact test for expected values < 3) for the comparison of proportions. The χ2 test or Fisher’s exact test was used to analyze categorical variables. The Mann–Whitney U and Wilcoxon W tests were used for variables that did not follow a normal distribution. p-value < 0.050 was considered statistically significant. Statistical analyses were performed using SPSS® software version 18 (IBM, Armonk, NY, USA).
Results
Baseline characteristics
A total of 108 patients were assessed for eligibility between August 2003 and August 2008. Of these, 24 were excluded for various reasons. Therefore, 84 patients were randomized (42 patients per group). Five patients died during the in-hospital postoperative period. These patients were not included in the analysis of DGE incidence; therefore, 79 patients were analyzed (40 in the study group and 39 in the control group). The flowchart of the study is shown in Fig. 1, and the baseline patient characteristics are shown in Table 1. The biliary tract was drained only in 15/84 patients (18%), with no differences between the two groups.
Primary efficacy endpoint
The incidence of DGE was 50% (20/40, 95% confidence interval (CI): 35–65%) in the study group and 62% (24/39, 95% CI: 46–75%) in the control group (p = 0.260).
DGE was associated with a longer hospital stay in both groups. The scintigraphy studies performed before surgery and at the 5th week after the intervention showed a mean (SD) percentage of radiotracer retention at 90 min of 35% (2.7) and 41% (2.9), respectively.
No significant differences were observed between the groups regarding the percentage of radiotracer retention at the preoperative study (39.9% and 31.2%, respectively) or the 5th-week post-surgery study (42.6% vs. 39.6%). Finally, we did not observe significant differences when comparing pre- and postoperative scintigraphy studies according to different DGE degrees.
Secondary efficacy endpoints
No differences were observed in the length of hospital stay between both groups (Table 2).
Regarding the anthropometric measurements, the weight before the disease was lower in the study group. Progressive weight loss was observed in both groups from the beginning of the disease until the 6th postoperative month. The upper arm circumference at 5 weeks post-surgery was greater in the control group than in the study group (26 cm vs. 28 cm, p = 0.030). The triceps fold measurement was greater in the control group at the 5th week (13 mm vs. 16 mm, p = 0.020) and 6th month (12 mm vs. 16 mm, p = 0.021) post-surgery than in the study group.
No significant differences were observed in the analytical values related to nutritional status.
The QoL questionnaire QLQ-PAN26 did not show differences between both groups either preoperatively or 6 months after surgery. During the 6-month follow-up period, 41 patients received chemotherapy, with no differences between both groups (p = 0.162). Additionally, 38 patients received radiotherapy, with no significant differences between both groups (p = 0.251) (Table 2).
Safety endpoint
The postoperative mortality rates were 4.8% (2/42) and 7.1% (3/42) in the study and control groups. Two patients died due to hemoperitoneum, two due to intra-abdominal sepsis and residual pancreatitis, and one due to acute myocardial infarction. No significant difference in mortality was observed between both groups.
Of 84 patients, 45 (53.6%) developed morbidity. However, no difference in overall morbidity was observed between both groups.
A greater incidence of pancreatic fistula was recorded in patients with invaginated anastomosis than in those with duct-to-mucosa anastomosis (17% vs. 12%). In addition, postoperative bleeding (percentage) was higher in the study group than in the control group; however, the difference was insignificant.
The percentage of reoperations was higher in the control group than in the study group; however, the difference was insignificant. Eight (10.1%) of 79 patients were reoperated on: three in the study group and five in the control group.
Discussion
Although the incidence of DGE was lower in patients who underwent classical Whipple (50%) than in those who underwent PPPD (62%), this difference was not significant.
Nevertheless, the incidence observed is remarkable, which we could justify for several reasons. First, during the study period, we adopted a more conservative approach to postoperative care. Thus, the nasogastric tube was maintained until 24–48 h after surgery and was removed only if the gastric aspirate was low. Second, since 2010, we have been performing antecolic gastrojejunal anastomoses [28], which has led to an improvement in DGE in these patients. Therefore, we are currently more active in initiating oral feeding, and we have also incorporated the Enhanced Recovery After Surgery (ERAS) program as part of our usual clinical practice to shorten postoperative hospital stay [29].
As mentioned above, no differences in DGE were observed when the two techniques were compared. However, several reflections must be made. In our study, we were unable to safely complete pancreatoduodenectomy with pylorus preservation in nine patients, and a classical Whipple had to be performed. Some patients had massive tumors, jeopardizing the oncological acceptability of resection. In other cases, the risk of duodenal ischemia forced us to perform gastrectomy. Similarly, Lin et al. [5] performed gastrectomy on five patients, and Tran et al. [30] on two. Tran et al. also found a higher percentage of affected tumor margins in the PPPD group than in the classical Whipple group (19 (26%) vs. 12 (17%), p = 0.023). Regarding these differences, there was a higher percentage of peripancreatic circumferential margins, defined as the dorsal resection margin (peripancreatic fat and fascia of Treitz) or beyond the anterior pancreatic parenchyma anteriorly (peripancreatic fat, mesenteric base of the transverse colon, or posterior peritoneum of the lesser sac) in the PPPD group when compared to the classical Whipple group, and one patient presented an affected duodenal margin. In addition, the PPPD group had a higher number of resections with affected margins [30]. Thus, in our opinion, the classical Whipple technique should be adopted as the first choice since it has the same morbidity rate and can be performed in all patients without the fear of duodenal ischemia. In addition, the risk of a positive margin in pylorus preservation should be considered.
RCTs published to date do not help decide which of these two techniques is better at reducing DGE incidence. In the late 1990s, Paquet et al. [7] and Wenger et al. [31] showed that PPPD was superior to the classical Whipple procedure in terms of better nutritional and endocrine recovery, and postoperative QoL. However, in 1999, Lin et al. [5] reported a higher incidence of DGE in patients who underwent PPPD. Subsequently, in the 2000s, Tran et al. [30] and Seiler et al. [32] published similar results between the two techniques in terms of postoperative morbidity and DGE. In 2008, Srinarmwong et al. [8] reported that PPPD was associated with a higher incidence of DGE. Finally, in 2015, Taher et al. [33] reported similar morbidity rates between the two techniques. Meta-analyses comparing the classical Whipple procedure and PPPD [34,35,36,37,38] also failed to demonstrate a clear difference in DGE incidence between the two techniques.
In recent years, several groups have advocated pyloric ring resection as a measure to improve DGE. Several RCTs [39,40,41,42] and meta-analyses [36,37,38, 43, 44] that compared pyloric ring preservation with resection have been published. The last two meta-analyses comparing pyloric ring resection and preservation in pancreatoduodenectomy indicated that pyloric resection is superior to pyloric preservation in terms of DGE [37, 38]. However, evidence in this regard is unclear.
The debate over a technique that provides the least DGE is still ongoing; the proof of this is the publication of two recent articles based on German and American data. In this sense, a German study on DGE risk factors was based on a record of more than 5000 patients [45] without being able to demonstrate differences between the two techniques. Notably, PPPD was the method of choice in most patients (70.4%). A recent publication of a large American study of more than 15,000 patients [46] revealed the need for continued investigation of the factors responsible for greater DGE. The authors created the PrEDICT-DGE score to identify patients at high risk for DGE and help guide perioperative management. Some procedures, such as concurrent adhesion, feeding jejunostomy, vein graft vascular reconstruction, or pancreatic invagination anastomosis, were identified as independent factors associated with DGE. Based on their findings, the author suggested that classical Whipple with duct-to-mucosa pancreatojejunostomy technique should be considered the primary surgical approach in high-risk DGE patients.
Nutritional status
The patient’s nutritional status and risk assessment of postoperative malnutrition should be part of the usual clinical practice before any pancreatic surgery, as recommended by the ISGPS [14]. The available data do not show any definitive nutritional advantages for a specific type of gastrointestinal reconstruction technique after pancreatoduodenectomy [42, 47, 48]. Therefore, a preoperative evaluation of patients undergoing pancreatic surgery should be performed, incorporating, among others, the percentage of body weight loss over time and body mass index (BMI) [40]. However, to date, no other group has investigated anthropometric changes after pancreatic surgery in relation to the type of gastroenteric reconstruction. We designed an RCT to evaluate postoperative DGE and analyze the nutritional status of patients after pancreatic surgery, with reference to pyloric preservation.
Maintaining appropriate nutritional support in patients with acute and chronic illnesses is a fundamental part of standard medical and surgical care. Malnourished patients have poorer clinical outcomes and higher morbidity and infection rates, and demand more healthcare resources than well-nourished patients [49]. Gastroparesis is one of the most under-diagnosed problems in patients with cancer and is often overlooked as a potential etiology of chronic nausea and vomiting. The exact prevalence of DGE is unknown; however, it is generally recognized that gastroparesis is common among patients with upper gastrointestinal tract tumors [50, 51] and after surgical treatment. A diagnosis of DGE is important in cancer and postoperative patients because the consequences of malignancy-associated gastroparesis can be serious, particularly in the context of other common problems that affect nutrition and fluid-electrolyte balance. Weight loss, anthropometric measurements, and various analytical variables have been associated with postoperative morbidity [14, 52,53,54,55]. The 5th-week postoperative evaluation showed superior anthropometric measurements in the PPPD group than in the study group in both the upper arm circumference and triceps fold (Fig. 2). However, at 6 months, these differences persisted only for the triceps fold measurement. We did not find a valid explanation for these differences; anthropometric changes likely respond to small modifications in the body constitution, which could result from the surgical technique. However, future studies should assess the anthropometric changes during the postoperative period of pancreatoduodenectomies. There were no differences in weight loss, BMI, or any of the laboratory variables analyzed between the study groups.
Isotope study of gastric emptying
Currently, gastric emptying scintigraphy following a standardized solid meal or liquid-phase gastric emptying (with 99mTc-radiolabeled pertechnetate mixed in orange juice) is the gold standard for diagnosing gastroparesis [25]. It can also be used to monitor the effectiveness of prokinetic therapy, although repeated exposure to radiation may be a limitation [56, 57]. Isotope studies after pancreatoduodenectomy have been used for years to assess DGE after pyloric preservation [57,58,59,60,61]; however, they have never been used in an RCT comparing both pancreatoduodenectomy techniques.
Although some authors [58] observed no differences in scintigraphy between both types of pancreatoduodenectomies, others [59] observed that more severe cases of DGE were associated with a higher percentage of residual radioactivity in the stomach after 120 min. Additionally, a more recent study [60] showed that scintigraphy performed on the 10th day after pancreatoduodenectomy had a better correlation with clinically relevant DGE than scintigraphy performed on the 21st day. In this study, we performed scintigraphy preoperatively and at 5 weeks postoperatively to observe long-term changes in gastric emptying. We did not observe differences in the retention of radiotracers between the study groups, nor did we find greater retention of radiotracers in patients with DGE. However, we observed a higher percentage of radiotracers in patients with severe DGE, although it was not significant.
Limitations
The first limitation of this study is the amount of time elapsed since we obtained our data, given that the analysis and presentation of the results were completed in 2010. Surgical practice and postoperative care have changed over the past 10 years. However, the surgical details of resection and reconstruction are essentially the same, and the postoperative morbidity remains similar. Furthermore, the study design followed the scientific method transparently and rigorously. Since new DGE concepts have been defined, and in light of recent publications that presented this problem regarding the magnitude of gastric resection, we thought it was necessary to report our results, which, despite being old, are reliable. As in other relevant studies, the timelessness of the problem is obvious, and, as previous meta-analyses advise, more RCTs should be performed to elucidate this. Second, the surgeons were responsible for the patients’ postoperative care. Masking was not possible because the investigators knew each patient’s randomization group. Given the results of this study, it does not seem necessary to preserve the pylorus during pancreatoduodenectomy since it did not provide any additional benefit. A long-term evaluation of the effect of PPPD was not performed and may be an endpoint to be pursued in future studies. Furthermore, a higher percentage of these patients died during the follow-up, making the long-term effect difficult to analyze. Another drawback is that the QoL PAN-26 results cannot be presented in detail because only the total score was collected in the database.
Conclusion
The incidence and severity of DGE did not differ between patients who underwent classical Whipple and those who underwent PPPD. Some anthropometric measurements may indicate better recovery with PPPD; however, both surgical pancreatoduodenectomy techniques were similar in terms of morbidity and mortality, nutritional status, and QoL. Therefore, in our opinion, the classical Whipple procedure should be the technique of choice because it can be performed in all patients without the fear of duodenal ischemia.
Data and/or code availability
The data sets used and/or analyzed during the study will be available from the corresponding author upon reasonable request.
References
Christians KK, Tsai S, Tolat PP, Evans DB (2013) Critical steps for pancreaticoduodenectomy in the setting of pancreatic adenocarcinoma. J Surg Oncol 107(1):33–38
Katz MHG, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA et al (2009) Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 16(July 2008):836–47
Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR et al (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142(5):761–768
Traverso LW, Freeny PC (1989) Pancreaticoduodenectomy. The importance of preserving hepatic blood flow to prevent biliary fistula. Am Surg. 55(7):421–6
Lin PW, Lin YJ (1999) Prospective randomized comparison between pyloruspreserving and standard pancreaticoduodenectomy. Br J Surg 86(5):603–607
Bloechle C, Broering DC, Latuske C, Latuske M, Schrenc T, Izbicki JR, et al. (1999) Prospektiv-randomisierter Vergleich zur Evaluation der Lebens-qualitätnach partieller Pankreatoduodenektomie nach Whippie und pyloruserhaltender Pankreatoduodenektomie nach Longmire-Traverso bei periampullärem Karzinom Prospective randomized study to eval. Langenbecks Arch Surg.;(Forumband):661–5
Paquet K-J (1998) Vergleich der partiellen Duodenopankreat- mit der pyloruserhaltenden Zephaloduo- denopankreatektomie ± eine prospektiv kontrollierte, randomi- sierte Langzeitstudie. chir gastroenterol. 14:54–8
Srinarmwong C, Luechakiettisak P, Prasitvilai W (2008) Standard Whipple’s operation versus pylorus preserving pancreaticoduodenectomy: a randomized controlled trial study. J Med Assoc Thail 91(5):693–698
Taher M, Khan Z, Chowdhury M, Nur-E-Elahi M, Chowdhury A, Faruque M et al (2015) Pylorus preserving pancreaticoduodenectomy vs standard Whipple’s procedure in case of carcinoma head of the pancreas and periampullary carcinoma. Mymensingh Med J. 24(2):319–25
Diener MK, Fitzmaurice C, Schwarzer G, Seiler CM, Hüttner FJ, Antes G et al (2014) Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev 11;11(11):CD006053. https://doi.org/10.1002/14651858.CD006053.pub5
Yang C, Wu HS, Chen XL, Wang CY, Gou SM, Xiao J et al (2014) Pylorus-preserving versus pylorus-resecting pancreaticoduodenectomy for periampullary and pancreatic carcinoma: a meta-analysis. PLoS One 6;9(3):e90316. https://doi.org/10.1371/journal.pone.0090316
Hüttner F, Fitzmaurice C, Schwarzer G, Seiler CMCCMC, Antes G, Büchler MW et al (2016) Pylorus-preserving pancreaticoduodenectomy (ppWhipple) versus pancreaticoduodenectomy (classicWhipple) for surgical treatment of periampullary and pancreatic carcinoma (Review). Cochrane Database Syst Rev. 2:CD006053
Probst P, Hüttner FJ, Meydan Ö, Abu Hilal M, Adham M, Barreto SG et al (2021) Evidence map of pancreatic surgery–a living systematic review with meta-analyses by the International Study Group of Pancreatic Surgery (ISGPS). Surg (United States) 170(5):1517–1524
Gianotti L, Besselink MG, Sandini M, Hackert T, Conlon K, Gerritsen A et al (2018) Nutritional support and therapy in pancreatic surgery: a position paper of the International Study Group on Pancreatic Surgery (ISGPS). Surg (United States) 164(5):1035–1048
Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P (2008) Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 148(4):295–309
Tani M, Kawai M, Hirono S, Okada KI, Miyazawa M, Shimizu A et al (2014) Randomized clinical trial of isolated Roux-en-Y versus conventional reconstruction after pancreaticoduodenectomy. Br J Surg 101(9):1084–1091
Busquets J, Fabregat J, Jorba R, Peláez N, García-Borobia F, Masuet C, et al. [Surgical treatment of pancreatic adenocarcinoma by cephalic duodenopancreatectomy (Part 1). Post-surgical complications in 204 cases in a reference hospital]. Cirugía española [Internet]. 2010 Nov [cited 2015 Jun 17];88(5):299–307. Available from: http://www.sciencedirect.com/science/article/pii/S0009739X10002496
Fabregat J, Busquets J, Peláez N, Jorba R, García-Borobia F, Masuet C, et al. [Surgical treatment of pancreatic adenocarcinoma using cephalic duodenopancreatectomy (Part 2). Long term follow up after 204 cases]. Cirugía española [Internet]. 2010 Dec [cited 2015 Jun 17];88(6):374–82. Available from: http://www.sciencedirect.com/science/article/pii/S0009739X10003532
Yeo CJ, Barry MK, Sauter PK, Sostre S, Lillemoe KD, Pitt HA et al (1993) Erythromycin accelerates gastric emptying after pancreatico duodenectomy. Ann Surg 218(3):229–237
van Berge Henegouwen MI, van Gulik TM, Akkermans LM, Jansen JB, Gouma DJ. (1997) The effect of octreotide on gastric emptying at a dosage used to prevent complications after pancreatic surgery: a randomised, placebo controlled study in volunteers. Gut 41(6):758–762. https://doi.org/10.1136/gut.41.6.758
Park JS, Hwang HK, Kim JK, Il Cho S, Yoon DS, Lee WJ et al (2009) Clinical validation and risk factors for delayed gastric emptying based on the International Study Group of Pancreatic Surgery (ISGPS) Classification. Surgery [Internet] 146(5):882–7. https://doi.org/10.1016/j.surg.2009.05.012
Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH et al (2008) Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol 103(3):753–763
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205-213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J et al (2005) International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138(1):8–13. https://doi.org/10.1016/j.surg.2005.05.001
Feinberg J, Nielsen EE, Gluud C, Lindschou J, Kondrup J, Jakobsen JC. (2015) Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2015(3)
Blackburn GL, Brisitan BR, Maini BS, Schlamm HT, Smith MF (1977) Nutritional and metabolic assessment of the hospitalized patient. J Parenter Enter Nutr. 1(1):11–21
Heerkens HD, van Berkel L, Tseng DSJ, Monninkhof EM, van Santvoort HC, Hagendoorn J et al (2018) Long-term health-related quality of life after pancreatic resection for malignancy in patients with and without severe postoperative complications. Hpb [Internet]. 20(2):188–95. https://doi.org/10.1016/j.hpb.2017.09.003
Busquets J, Martín S, Fabregat J, Secanella L, Pelaez N, Ramos E (2019) Randomized trial of two types of gastrojejunostomy after pancreatoduodenectomy and risk of delayed gastric emptying (PAUDA trial). Br J Surg [Internet]. 46–54. Available from: https://onlinelibrary.wiley.com/doi/abs/https://doi.org/10.1002/bjs.11023?af=R
Barton JG (2016) Enhanced recovery pathways in pancreatic surgery. Surg Clin North Am [Internet]. 96(6):1301–12. https://doi.org/10.1016/j.suc.2016.07.003
Tran KT, Smeenk HG, van Eijck CH, Kazemier G, Hop WC, Greve JW et al (2004) Pylorus preserving pancreaticoduodenectomy versus standard Whipple procedure: a prospective, randomized, multicenter analysis of 170 patients with pancreatic and periampullary tumors. Ann Surg 240(5):738–745. https://doi.org/10.1097/01.sla.0000143248.71964.29
Wenger FA, Jacobi CA, Haubold K, Zieren HU, Müller JM (1999) Gastrointestinale Lebensqualität nach Duodenopankreatektomie beim Pankreascarcinom. Vorläufige Ergebnisse einer prospektiv-randomisierten Studie: PD vs PPPD [Gastrointestinal quality of life after duodenopancreatectomy in pancreatic carcinoma. Preliminary results of a prospective randomized study: pancreatoduodenectomy or pylorus-preserving pancreatoduodenectomy]. Chirurg 70(12):1454-1459. German. https://doi.org/10.1007/pl00002580
Seiler CA, Wagner M, Bachmann T, Redaelli CA, Schmied B, Uhl W et al (2005) Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection - long term results. Br J Surg 92(5):547–556
Taher M, Khan Z, Chowdhury M, Nur-E-Elahi M, Chowdhury A, Faruque M et al (2015) Pylorus preserving pancreaticoduodenectomy vs. standard Whipple’s procedure in case of carcinoma head of the pancreas and periampullary carcinoma. Mymensingh Med J. 24(2):319–25
Karanicolas PJ, Davies E, Kunz R, Briel M, Koka HP, Payne DM et al (2007) The pylorus: take it or leave it? Systematic review and meta-analysis of pylorus-preserving versus standard Whipple pancreaticoduodenectomy for pancreatic or periampullary cancer. Ann Surg Oncol 14:1825–1834
Diener MK, Knaebel H-P, Heukaufer C, Antes G, Büchler MW, Seiler CM (2007) A systematic review and meta-analysis of pylorus-preserving versus classical pancreaticoduodenectomy for surgical treatment of periampullary and pancreatic carcinoma. Ann Surg 245:187–200
Lin L, Wu L, Li B, Zhou Y, Xu D (2014) A case-matched comparison and meta-analysis comparing pylorus-resecting pancreaticoduodenectomy with pylorus-preserving pancreaticoduodenectomy for the incidence of postoperative delayed gastric emptying. Hpb [Internet]. 17(4):337–43. https://doi.org/10.1111/hpb.12358
Hanna M, Gadde R, Allen C, Meizoso J, Sleeman D, Livingstone AS et al (2016) Delayed gastric emptying after pancreaticoduodenectomy. J Surg Res 202(2):380–388
Varghese C, Bhat S, Wang TH, O'Grady G, Pandanaboyana S (2021) Impact of gastric resection and enteric anastomotic configuration on delayed gastric emptying after pancreaticoduodenectomy: a network meta-analysis of randomized trials. BJS Open 7;5(3):zrab035. https://doi.org/10.1093/bjsopen/zrab035
Klaiber U, Probst P, Hüttner FJ, Bruckner T, Strobel O, Diener MK et al (2020) Randomized trial of pylorus-preserving vs. pylorus-resecting pancreatoduodenectomy: long-term morbidity and quality of life. J Gastrointest Surg. 24(2):341–52
Hackert T, Probst P, Knebel P, Doerr-Harim C, Bruckner T, Klaiber U et al (2018) Pylorus resection does not reduce delayed gastric emptying after partial pancreatoduodenectomy a blinded randomized controlled trial (PROPP Study, DRKS00004191). Ann Surg 267(6):1021–1027
Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, Uchiyama K et al (2011) Pylorus ring resection reduces delayed gastric emptying in patients undergoing pancreatoduodenectomy: a prospective, randomized, controlled trial of pylorus-resecting versus pylorus-preserving pancreatoduodenectomy. Ann Surg 253(3):495–501
Matsumoto I, Shinzeki M, Asari S, Goto T, Shirakawa S, Ajiki T et al (2014) A prospective randomized comparison between pylorus- and subtotal stomach-preserving pancreatoduodenectomy on postoperative delayed gastric emptying occurrence and long-term nutritional status. J Surg Oncol 109(7):690–696
Li W, Liu X, Yang C, Fu L, Liang P, Zhu J et al (2019) No increase in delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: a meta-analysis of RCTs. https://www.semanticscholar.org/paper/No-increase-in-delayed-gastric-emptying-after-a-of-Li-Liu/1328e58be26bf6c45ce36ebdb9aeabb2a533f1b9
Klaiber U, Probst P, Strobel O, Michalski CW, Dörr-Harim C, Diener MK et al (2018) Meta-analysis of delayed gastric emptying after pylorus-preserving versus pylorus-resecting pancreatoduodenectomy. Br J Surg 105(4):339–349
Fahlbusch T, Luu AM, Höhn P, Klinger C, Werner J, Keck T et al (2022) Impact of pylorus preservation on delayed gastric emptying after pancreaticoduodenectomy—analysis of 5,000 patients based on the German StuDoQ|Pancreas-Registry. Gland Surg 11(1):67–76
Werba G, Sparks AD, Lin PP, Johnson LB, Vaziri K (2021) The PrEDICT-DGE score as a simple preoperative screening tool identifies patients at increased risk for delayed gastric emptying after pancreaticoduodenectomy. HPB 24(1):30–39
Kawai M, Tani M, Hirono S, Okada KI, Miyazawa M, Yamaue H (2014) Pylorus-resecting pancreaticoduodenectomy offers long-term outcomes similar to those of pylorus-preserving pancreaticoduodenectomy: results of a prospective study. World J Surg 38(6):1476–1483
Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, Uchiyama K et al (2011) Pylorus ring resection reduces delayed gastric emptying in patients undergoing pancreatoduodenectomy. Ann Surg 253(3):495–501
Allard JP, Keller H, Jeejeebhoy KN, Laporte M, Duerksen DR, Gramlich L et al (2016) Decline in nutritional status is associated with prolonged length of stay in hospitalized patients admitted for 7 days or more: a prospective cohort study. Clin Nutr 35(1):144–152
Schraml FV, Krueger WH (2005) Presentation of gastric carcinoma on a radionuclide gastric-emptying study. Clin Nucl Med 30(8):574–576
Leung VKS, Kan PS, Lai MS (2003) Cholangiocarcinoma presenting as pseudoachalasia and gastroparesis. Hong Kong Med J 9(4):296–298
Jimenez RE, Fernandez-del Castillo C, Rattner DW, Chang Y, Warshaw AL (2000) Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg 231(3):293–300
Ohtsuka T, Tanaka M, Miyazaki K (2006) Gastrointestinal function and quality of life after pylorus-preserving pancreatoduodenectomy. J Hepatobiliary Pancreat Surg 13(3):218–224
Klaiber U, Probst P, Knebel P, Contin P, Diener MK, Büchler MW et al (2015) Meta-analysis of complication rates for single-loop versus dual-loop (Roux-en-Y) with isolated pancreaticojejunostomy reconstruction after pancreaticoduodenectomy. Br J Surg 102(4):331–340. https://doi.org/10.1002/bjs.9703
Probst P, Haller S, Dörr-Harim C, Bruckner T, Ulrich A, Hackert T et al (2015) Nutritional risk in major abdominal surgery: protocol of a prospective observational trial to evaluate the prognostic value of different nutritional scores in pancreatic surgery. JMIR Res Protoc 16;4(4):e132. https://doi.org/10.2196/resprot.4567
Fich A, Neri M, Camilleri M, Kelly KA, Phillips S (1990) Stasis syndromes following gastric surgery: clinical and motility features of 60 symptomatic patients. J clin gastroenterol. 5:505–12
Kobayashi I, Miyachi M, Kanai M, Nagino M, Kondo S, Kamiya J (1988) Different gastric emptying of solid and liquid meals after pylorus-preserving pancreatoduodenectomy. Br J Surg 85:927–930
Williamson RC, Bliouras N, Cooper MJ, Davies ER (1993) Gastric emptying and enterogastric reflux after conservative and conventional pancreatoduodenectomy. Surgery 114(1):82–86
Van Samkar G, Eshuis WJ, Lemmers M, Gouma DJ, Bennink RJ, Hollmann MW et al (2013) Value of scintigraphy for assessing delayed gastric emptying after pancreatic surgery. World J Surg 37(12):2911–2917
Samaddar A, Kaman L, Dahiya D, Bhattachyarya A, Sinha SK (2017) Objective assessment of delayed gastric emptying using gastric scintigraphy in post pancreaticoduodenectomy patients. ANZ J Surg 87(9):E80–E84
Hishinuma S, Ogata Y, Matsui J, Ozawa I (1999) Evaluation of pylorus-preserving pancreatoduodenectomy with the Imanaga reconstruction by hepatobiliary and gastrointestinal dual scintigraphy. Br J Surg 86(10):1306–1311
Acknowledgements
We would like to thank Dr. C. Masuet (Preventive Medicine and Public Health Service of the Hospital Universitari de Bellvitge) for providing advice on statistical analysis. We would also like to thank the IDIBELL Foundation and the CERCA Program/Generalitat de Catalunya for the institutional support provided.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research did not receive any specific grants from any funding agency in the public, commercial, or not-for-profit sectors. This study was funded by the investigator’s personal funds. The costs of the surgical procedures and complementary tests were paid by the Catalan Institute of Health, and no additional costs were incurred. The investigators did not receive any economic compensation to conduct the study. Therefore, the study design, data collection, analysis, interpretation, and the original draft’s writing, review, and editing were performed by members of the study team. The corresponding author had full access to all the study data and was responsible for submitting the paper for publication.
Author information
Authors and Affiliations
Contributions
Study conception and design: JA, JB, JF. Acquisition of data: NC, SM, NP, JB, MB. Analysis and interpretation of data: LS, JB, SM, SV. Drafting of the manuscript: TC. Critical revision of the manuscript: JB, SM, SV, TC.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by the Ethics Committee (185/03) of the Bellvitge University Hospital, University of Barcelona, and it was registered at ClinicalTrials.gov (QUANUPAD Trial; NCT03984734). The study complied with the criteria set by the Declaration of Helsinki (revised on WMA 64th General Assembly, Fortaleza, Brazil, October 2013), Good Clinical Practice (GCP) standards, and applicable regulations.
Consent to participate and/or consent for publication
Written informed consent was obtained from all the patients included.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
S. Videla and J. Fabregat are joint senior authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Busquets, J., Martín, S., Secanella, L. et al. Delayed gastric emptying after classical Whipple or pylorus-preserving pancreatoduodenectomy: a randomized clinical trial (QUANUPAD). Langenbecks Arch Surg 407, 2247–2258 (2022). https://doi.org/10.1007/s00423-022-02583-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02583-9