Abstract
Aims
Adjuvant chemotherapy for resected rectal cancer is widely used. However, studies on adjuvant treatment following neoadjuvant chemoradiotherapy (CRT) and total mesorectal excision (TME) have yielded conflicting results. Recent studies have focused on adding oxaliplatin to both preoperative and postoperative therapy, making it difficult to assess the impact of adjuvant oxaliplatin alone. This study was aimed at determining the impact of (i) any adjuvant treatment and (ii) oxaliplatin-containing adjuvant treatment on disease-free survival in CRT-pretreated, R0-resected rectal cancer patients.
Method
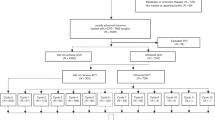
Patients undergoing R0 TME following 5-fluorouracil (5FU)-only-based CRT between January 1, 2008, and December 31, 2010, were selected from a nationwide registry. After propensity score matching (PSM), comparison of disease-free survival (DFS) using Kaplan-Meier analysis and log-rank test was performed in (i) patients receiving no vs. any adjuvant treatment and (ii) patients treated with adjuvant 5FU/capecitabine without vs. with oxaliplatin.
Results
Out of 1497 patients, 520 matched pairs were generated for analysis of no vs. any adjuvant treatment. Mean DFS was significantly prolonged with adjuvant treatment (81.8 ± 2.06 vs. 70.1 ± 3.02 months, p < 0.001). One hundred forty-eight matched pairs were available for analysis of adjuvant therapy with or without oxaliplatin, showing no improvement in DFS in patients receiving oxaliplatin (76.9 ± 4.12 vs. 79.3 ± 4.44 months, p = 0.254). Local recurrence rate was not significantly different between groups in either analysis.
Conclusion
In this cohort of rectal cancer patients treated with neoadjuvant CRT and TME surgery under routine conditions, adjuvant chemotherapy significantly improved DFS. No benefit was observed for the addition of oxaliplatin to adjuvant chemotherapy in this setting.



Similar content being viewed by others
References
Moertel CG, Fleming TR, Macdonald JS et al (1990) Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322:352–358
O'Connell MJ, Mailliard JA, Kahn MJ et al (1997) Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 15:246–250
Gastrointestinal Tumor Study Group (1985) Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med 312:1465–1472
Sauer R, Becker H, Hohenberger W et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
Sauer R, Liersch T, Merkel S et al (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30:1926–1933
Kapiteijn E, Marijnen CA, Nagtegaal ID et al (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638–646
Breugom AJ, van Gijn W, Muller EW et al (2014) Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomised phase III trial. Ann Oncol 26:696–701
Glynne-Jones R, Counsell N, Quirke P et al (2014) Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol 25:1356–1362
Sainato A, Nunzia CL, Valentina VV et al (2014) No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol 113:223–229
Bosset J, Calais G, Mineur L et al (2014) Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 15:184–190
André T, Boni C, Mounedji-Boudiaf L et al (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343–2351
Kuebler JP, Wieand HS, O'Connell MJ et al (2007) Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 25:2198–2204
Yothers G, O'Connell MJ, Allegra CJ et al (2011) Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 29:3768–3774
Hong YS, Nam B, Kim K et al (2014) Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol 15:1245–1253
Schmoll HJ, Haustermans K, Price TJ et al. (2014) Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: disease-free survival results at interim analysis. J Clin Oncol 32(suppl; abstr 3501)
Rodel C, Liersch T, Fietkau R et al (2015) Preoperative chemoradiotherapy and postoperative chemotherapy with 5-fluorouracil and oxaliplatin versus 5-fluorouracil alone in locally advanced rectal cancer: results of the German CAO/ARO/AIO-04 randomized phase III trial. Lancet Oncol 16:979–989
Rosenbaum PR, Rubin DB (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70:41–55
Trojano M, Pellegrini F, Paolicelli D, Fuiani A, Di Renzo V (2009) Observational studies: propensity score analysis of non-randomized data. Int MS J 16:90–97
Hermanek P, Hohenberger W, Klimpfinger M, Köckerling F, Papadopoulos T (2003) The pathological assessment of mesorectal excision: implications for further treatment and quality management. Int J Colorect Dis 18:335–341
Fisher B, Wolmark N, Rockette H et al (1988) Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Nat Cancer Inst 80:21–29
Petersen SH, Harling H, Kirkeby LT, Wille-Jørgensen P, Mocellin S (2012) Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev 3:CD004078
International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators (1995) Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet 345:939–944
Breugom AJ, Swets M, Bosset J et al (2015) Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 16:200–207
Bujko K, Glimelius B, Valentini V, Michalski W, Spalek M (2015) Postoperative chemotherapy in patients with rectal cancer receiving preoperative radio(chemo)therapy: a meta-analysis of randomized trials comparing surgery ± a fluoropyrimidine and surgery + a fluoropyrimidine ± oxaliplatin. Eur J Surg Oncol 41:713–723
Ahn DH, Wu C, Wei L et al (2015) The efficacy of adjuvant chemotherapy in patients with stage II/III resected rectal cancer treated with neoadjuvant chemoradiation therapy. Am J Clin Oncol. doi:10.1097/COC.0000000000000185
Petrelli F, Coinu A, Lonati V, Barni S (2015) A systematic review and meta-analysis of adjuvant chemotherapy after neoadjuvant treatment and surgery for rectal cancer. Int J Colorect Dis 30:447–457
Gresham G, Cheung WY, Speers C, Woods R, Kennecke H (2015) Time to adjuvant chemotherapy and survival outcomes among patients with stage 2 to 3 rectal cancer treated with preoperative chemoradiation. Clin Colorectal Cancer 14:41–45
Schrag D, Weiser MR, Goodman KA et al (2014) Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 32:513–518
Nilsson PJ, van Etten B, Hospers GAP et al (2013) Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer--the RAPIDO trial. BMC Cancer 13:279
Chemotherapy alone or chemotherapy plus radiation therapy in treating patients with locally advanced rectal cancer undergoing surgery. https://clinicaltrials.gov/ct2/show/NCT01515787. Accessed 11 Oct 2015
Author contributions
BG conceptualized and designed the study, was involved in data collection and analysis, and wrote the manuscript. HP was involved in designing the study, data collection, interpretation, and analysis and helped to write the manuscript. FB and FP were involved in data collection, interpretation, and analysis. RO did the statistical analysis and was involved in data processing for presentation. KR, IG, HL, and CBr were involved in data collection and interpretation, as well as data extraction and processing from the Quality Assurance database. CBe helped to integrate the reviewer’s comments into the revised version and was involved in collecting additional data for manuscript revision as well as the processing of these data for presentation. All authors approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that no financial and personal relationships with other people or organizations that could inappropriately influence (bias) their existing work. This includes, but is not limited to, employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
Funding
There has been no external funding for the conduct of the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Garlipp, B., Ptok, H., Benedix, F. et al. Adjuvant treatment for resected rectal cancer: impact of standard and intensified postoperative chemotherapy on disease-free survival in patients undergoing preoperative chemoradiation—a propensity score-matched analysis of an observational database. Langenbecks Arch Surg 401, 1179–1190 (2016). https://doi.org/10.1007/s00423-016-1530-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-016-1530-0