Abstract
Introduction
Sodium bicarbonate (NaHCO3) ingestion has been found to be ergogenic in high-intensity exercise that ranges from 1 to 10 min; however, limited studies have investigated high-intensity exercise beyond this duration.
Purpose
The present study aimed to determine the effect of NaHCO3 ingested using a carbohydrate hydrogel delivery system on 40 km time trial (TT) performance in trained male cyclists.
Methods
Fourteen trained male cyclists ingested 0.3 g kg−1 BM NaHCO3 (Maurten AB, Sweden) to determine individualised peak alkalosis, which established time of ingestion prior to exercise. Participants completed a 40 km familiarisation TT, and two 40 km experimental TTs after ingestion of either NaHCO3 or placebo in a randomised, double-blind, crossover design.
Results
NaHCO3 supplementation improved performance (mean improvement = 54.14 s ± 18.16 s; p = 0.002, g = 0.22) and increased blood buffering capacity prior to (HCO3− mean increase = 5.6 ± 0.2 mmol L−1, p < 0.001) and throughout exercise (f = 84.82, p < 0.001, pη2 = 0.87) compared to placebo. There were no differences in total gastrointestinal symptoms (GIS) between conditions either pre- (NaHCO3, 22 AU; Placebo, 44 AU; p = 0.088, r = 0.46) or post-exercise (NaHCO3, 76 AU; Placebo, 63 AU; p = 0.606, r = 0.14).
Conclusion
The present study suggests that ingesting NaHCO3 mini-tablets in a carbohydrate hydrogel can enhance 40 km TT performance in trained male cyclists, with minimal GIS. This ingestion strategy could therefore be considered by cyclists looking for a performance enhancing ergogenic aid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is considerable research evidence to support the ergogenic effects of sodium bicarbonate (NaHCO3) for high-intensity exercise performance across a variety of durations and distances. Traditionally, NaHCO3 is predominantly recommended for use in high intensity exercise up to 10 min in duration (Carr et al. 2011; McNaughton 1992; McNaughton et al. 2016). This buffering agent has been shown to enhance performance in a host of disciplines and durations, such as cross-fit athletes’ 2 km rowing performance (Martin et al. 2023), regional swimmers’ latter stages of repeated swimming sprints (Gough et al. 2023) and trained cyclists’ 4 km time trial (TT) performance (Hilton et al. 2020). Although there are several key physiological factors associated with skeletal muscle fatigue during high-intensity exercise, the most common consensus is the intra-muscular and extracellular accumulation of hydrogen (H+) ions (Carr et al. 2011; Requena et al. 2005). The ingestion of NaHCO3 increases blood bicarbonate concentration (HCO3−) which facilitates an enhanced H+ efflux from the intracellular space (Siegler et al. 2016). This subsequently enhances the muscle contractile capacity and glycolytic rate, which can delay the onset of fatigue and improve exercise performance (Requena et al. 2005).
The underlying mechanisms that enable performance improvements following the ingestion of NaHCO3 is best suited for high-intensity exercise that utilises a high proportion of energy via non-oxidative pathways (de Oliveira et al. 2021; Hilton et al. 2020; McNaughton et al. 2016). However, some studies have also shown performance enhancements following the ingestion of extracellular buffers in high-intensity exercise exceeding 10 min in duration. These buffering agents include sodium citrate (SC; Potteiger et al. 1996; Oopik et al., 2004), sodium phosphate (SP; Folland et al. 2008) and lactate supplementation (Morris et al. 2011). Despite the improved performance originated from these extracellular buffering agents, NaHCO3 is widely regarded as the most effective extracellular buffer (de Oliviera et al., 2021) and the most readily available. Research, therefore, needs to explore whether ingestion of NaHCO3 can also achieve similar performance enhancements during longer duration, high intensity exercise.
Earlier research on NaHCO3 on prolonged high intensity exercise by George and MacLaren (1988) observed a 17% performance improvement in time to exhaustion (TTE) when running on a treadmill at a velocity corresponding to 4 mmol L−1 of blood lactate following a 0.2 g kg−1 BM dose. This magnitude of improvement is similar to the work of McNaughton et al. (1999), where 0.3 g kg−1 BM NaHCO3 enhanced total work by 13.3% in a 1 h competitive cycling TT. Additionally, Egger et al., (2014) also observed improved TTE during cycling at 110% individual anaerobic threshold (IAT), following the ingestion of 0.3 g kg−1 BM of NaHCO3 in solution. Finally, in a more ecologically valid assessment of performance, Leach et al., (2023) studied the effects of pre-exercise NaHCO3 in the form of gelatin and enterically coated capsules on 16.1 km TT performance. These authors observed a 2.1% and 2.5% improvement respectively, in comparison to a placebo, using an individualised time to peak alkalosis (TTP) ingestion strategy (Miller et al. 2016). This differs from the previous research investigating NaHCO3 on prolonged high intensity exercise as historically studies tended to use standardised ingestion strategies of 60 min (Egger et al. 2014), 90 min (McNaughton et al. 1999), and 120 min (George and MacLaren 1988).
Despite the aforementioned research demonstrating performance enhancing effects with NaHCO3 ingested at standardised times, implementing an individualised ingestion strategy may further increase the ergogenic effects of NaHCO3 (Boegman et al. 2020). Pre-determining individualised ingestion strategies accounts for the inter-individual variation in time to peak HCO3− (Gough et al. 2017) and blood pH (Sparks et al. 2017), potentially increasing the performance enhancing effects of NaHCO3 ingestion (Boegman et al. 2020). Consequently, additional research is necessary to establish the effect of NaHCO3 on longer duration TT performance when ingested at TTP alkalosis.
The most utilised ingestion strategies of NaHCO3 are via solution (McNaughton et al. 2019) and gelatin capsules (Carr et al. 2011). However, gastrointestinal symptoms (GIS) are common with these modes (Hilton et al. 2020). Consequently, researchers and athletes have begun to utilise other modes of ingestion, such as enterically coated and delayed-release capsules (Hilton et al. 2019, 2020; Leach et al. 2023) and commercially available ingestion systems (Gough and Sparks 2024). As such, a new commercially available NaHCO3 product (Maurten Bicarb System) claims to reduce GIS and increase extracellular blood buffering capacity through the medium of NaHCO3 mini-tablets embedded within a carbohydrate (CHO) hydrogel. This enables the passage of the mini-tablets through the pyloric sphincter, reducing the interaction of NaHCO3 with stomach acid. A recent study has shown that this novel ingestion system significantly reduces GIS and extends the duration of potentially ergogenic alkalosis in comparison to NaHCO3 ingested in vegetarian capsules (Gough and Sparks 2024). However, the effects of NaHCO3 mini-tablets within a CHO hydrogel on TT performance is currently unclear. Therefore, the aim of the present study was to determine the effects of ingesting 0.3 g kg−1 BM NaHCO3 at TTP using a CHO hydrogel system on 40 km TT performance in trained male cyclists.
Methods
Participants
Fourteen, trained, male cyclists (age 43 ± 15 years; height 1.74 ± 0.68 m; body mass 75 ± 9 kg; body fat 15.1 ± 4.9%, VO2Peak 51.9 ± 6.4 mL kg−1 min−1, HRmax 185 ± 8 b min−1, WPeak 383 ± 49 W) were recruited for this study. The sample size was determined using G*Power (v3.1.9.6, Kiel University, Germany) for within factor analysis, assuming a 0.05 error probability and 0.80 power. The analysis revealed that twelve participants would be adequate to detect changes in performance with a small effect size (0.20). The participants cycled > 3 day week−1 and > 180 km week−1 and had a minimum of two years of cycling experience. Each participant regularly competed in cycling TTs. Based on these characteristics, and in accordance with performance criteria from McKay et al., (2021), participants were classed as “trained”. Individuals with clinically diagnosed GIS-related disorders, hypertension, renal impairment, and individuals who were currently ingesting nutritional buffering supplements or were currently on any type of medication were excluded from the study. Ethical approval (ETH2223-0084) was secured from the institutional research ethics committee whilst written and verbal informed consent was obtained from each participant prior to participation. All procedures were conducted in accordance with the Declaration of Helsinki (2013).
Experimental design
In a randomised, double-blind, crossover design, participants attended Edge Hill University’s Physiology Laboratory on five separate occasions separated by at least 48 h. The first visit measured time to peak blood alkalosis (Miller et al. 2016). Following this, the second visit measured body composition (BodPod®, Cosmed, Italy) and the participant’s VO2Peak. The third visit comprised of a familiarisation 40 km TT. Finally, the last two visits were allocated to two 40 km TTs, in which the participants ingested either a 0.3 g kg−1 BM dose of NaHCO3 (Maurten AB, Gothenburg, Sweden) or a taste and texture matched placebo. To determine the effectiveness of the blinding, participants were asked whether they believed they had ingested NaHCO3 or placebo. Dietary intake was registered in a food diary 24 h prior to the first of the trials and participants were asked to replicate this in all subsequent trials. Each participant arrived at the laboratory 2 h post-prandial, whilst each laboratory visit was arranged to occur at the same time of day (± 1 h) to limit the physiological effects of circadian variations (Reilly 1990). Finally, participants refrained from alcohol consumption and strenuous activity 24 h before each experimental trial.
Ingestion strategy
The ingestion strategy involved the consumption of 40 g of CHO hydrogel mixed with 200 mL of water, and 0.3 g kg−1 BM of sodium bicarbonate mini-tablets for the NaHCO3 trials (Maurten Bicarb System, Maurten AB, Gothenburg, Sweden). For the placebo, the water and hydrogel mixture had 6.50 g of finely crushed non-caloric sweetener (Sula®, Sula GmbH, Metelen, Germany) added in place of the NaHCO3 mini-tablets, to taste and texture match it. Once the CHO hydrogel was added to the water, it was mixed and left for 5 min, in accordance with the manufacturer’s instructions, then either the NaHCO3 or placebo were added, and it was ingested immediately.
Data Collection.
Peak oxygen uptake
The determination of VO2Peak was achieved by administering a ramp cycling test (Deb et al. 2018). The ramp test was conducted on an electromagnetically braked cycle ergometer (Lode Excalibur Sport, Groningen, the Netherlands), and consisted of a 5 min warm-up at 70 W at a self-selected cadence between 70 and 90 rpm, followed by an increase in workload of 30 W min−1 until volitional exhaustion. Breath-by-breath oxygen consumption (VO2), carbon dioxide (VCO2), minute ventilation (VE) and respiratory exchange ratio (RER) were measured continuously (Vyntus, Vyaire Medical, Illinois, USA). The determination of VO2Peak was defined as the highest 30 s rolling VO2 average of the ramp test. This occurred at volitional exhaustion when (a) RER > 1.15 and (b) rating of perceived exertion (RPE) > 18/20 AU (Midgley et al. 2007).
Time to peak (TTP) blood alkalosis
To determine the time to reach peak alkalosis, participants rested for 10 min before an initial blood sample was collected in a 95 μL heparin-coated glass capillary tube (Radiometer Medical Ltd, Copenhagen, Denmark). Participants then remained seated and ingested 0.3 g kg−1 NaHCO3. Post-ingestion 95 μL fingertip blood samples were taken every 10 min, up to 180 min (Gough et al. 2017), and were analysed immediately using a reliable and valid (Stadlbauer et al. 2011) blood gas analyser (Radiometer ABL800 BASIC, Copenhagen, Denmark) for blood HCO3− and pH. Peak alkalosis was defined as the greatest blood HCO3− concentration recorded within 180 min and subsequently determined the individualised timing of ingestion for the experimental trials. Peak HCO3− has previously been shown to be a more reliable measure of alkalosis than peak pH (Gough et al. 2017). During this time, participants were instructed to record any GIS on a 9-item, 10-point Likert Scale, (0 = no symptom, and 10 = severe symptom) following each blood sample (Carr et al. 2011). Symptoms on the 9-item scale included nausea, flatulence, stomach cramping, belching, stomach-ache, bowel urgency, diarrhoea, vomiting, and stomach bloating.
40 km time trials
Following a 10 min seated rest, an initial finger-tip blood sample (blood lactate, pH, HCO3−) was collected. After which, participants ingested either 0.3 g kg−1 NaHCO3 or a placebo, in a randomised order. Participants were instructed to record any GIS on the previously described 9-item, 10-point Likert scale pre-ingestion, pre-exercise, and post-exercise. Total GIS was defined as the number of individual symptom scores (Arbitrary Units; AU) per timepoint and per condition, whereby total aggregated GIS was characterised as the accumulation of individual symptoms per condition throughout both timepoints (pre-exercise and post exercise). After ingestion, participants remained rested until their individualised TTP alkalosis timepoint, after which another resting finger-tip blood sample (blood; pH, HCO3−) was drawn. Participants then completed a 5 min self-selected warm-up before starting the 40 km TT. An additional finger-tip blood sample was drawn at the end of the warm-up. All TT’s were completed using an air braked cycle ergometer (Wattbike Pro, Nottingham, UK), which began immediately following the post warm-up blood sample and were replicated in all subsequent trials.
Performance metrics including mean power, mean cadence, mean speed, and split time were recorded at 10, 20, 30, and 40 km of the TT, whilst total completion time was recorded as soon as the TT finished. Blood lactate (Lactate Pro 2 Analyser, Arkray, Japan), average heart rate (HR; Forerunner, Garmin, Olathe, Kansas, USA), RPE (6–20 scale; Borg, 2002), blood pH, HCO3−, and electrolytes (Na+, K+, Cl−, Ca2+) were measured at 10, 20, 30, and 40 km timepoints. Furthermore, at 9–10, 19–20, and 29–30 km, respiratory responses were recorded as previously described, by means of wearing a nosepiece and placing a mouthpiece in the participant’s mouth to facilitate intermittent sample collection. The TTs took place in a temperate controlled laboratory environment (temperature, 19 ± 2 °C, humidity, 45 ± 5%; pressure, 753 ± 9 mmHg). Throughout each TT, a fan (Clarke Air, Essex, UK) was placed 1.5 m away from the ergometer, to promote evaporative cooling. Participants received real time feedback on their heart rate and distance covered during each time trial, but were blinded from power-output, time-elapsed, and speed data. No verbal encouragement was provided during the TT’s.
Statistical analyses
Assessed variables were analysed for normality (Shapiro–Wilk Test) and homogeneity of variance (Mauchly Test), whereby violations were corrected, if necessary, via Greenhouse–Geisser adjustments. To analyse overall TT performance, a paired samples t-test was conducted, and effect sizes were calculated using Hedge’s g. Effect sizes were considered as: trivial (< 0.20), small (0.20–0.49), moderate (0.50–0.79) or large (> 0.80) (Cohen 1988). The assumption of normal distribution was violated for GIS, therefore, a Wilcoxon signed rank test was conducted as the non-parametric alternative. Effect sizes (r) for non-normally distributed data were calculated from z/n, with 0.10, 0.24, and 0.37 considered as small, medium, and large, respectively (Ivarsson et al. 2013). Additionally, a two-way repeated measures (condition x time) analysis of variance (ANOVA) was conducted using a Bonferroni correction to analyse changes in mean power, split time, mean cadence, mean speed, acid–base balance (blood HCO3− and blood pH), electrolytes (Na+, K+, Cl−, Ca2+), RPE, physiological variables (HR, blood lactate), and respiratory responses (VE, VO2, VCO2 and RER) during the experimental trials. Effect sizes for pη2 were interpreted as small (0.01), medium (0.06) and large (0.14) (Cohen 1988). Data are presented as mean ± standard deviation (SD), unless stated otherwise. Data were analysed using SPSS Statistics (Version 29, IBM SPSS Inc., Chicago, IL, USA), with significance set at p < 0.05.
Results
40 km tine trial performance
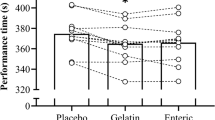
Overall TT performance (Figs. 1 and 2) was improved (mean improvement = 54.14 ± 18.16 s) following ingestion of 0.3 g kg−1 BM NaHCO3 compared to the placebo (total time, t = 3.75, p = 0.002, g = 0.22; overall mean power output, t = 3.72, p = 0.003, g = 0.21; mean power output, f = 13.83, p = 0.003, pη2 = 0.516; mean speed, f = 14.24, p = 0.02, pη2 = 0.002; split time, f = 13.88, p = 0.003, pη2 = 0.52). Twelve out of the fourteen participants performed better following NaHCO3 supplementation in comparison to the placebo (Fig. 1). There was no significant trial order effect (t = 0.91, p = 0.38, g = 0.07) and familiarisation performance times were not significantly different to placebo (mean difference (MD) = -36.21 s, p = 0.056, g = 0.14), suggesting minimal variability for the 40 km TT performance test and no significant placebo effect. A little over half (57%) of the participants correctly guessed which supplement was ingested. There was no significant condition*distance interaction effect between mean power output (f = 2.02, pη2 = 0.355, p = 0.355), mean speed (f = 2.22, pη2 = 0.377, p = 0.143), or split time (f = 2.15, p = 0.14, pη2 = 0.14), suggesting minimal differences in pacing strategy in the TTs between both conditions.
Mean ± SD split time (A), mean power (B) and mean speed (C). “∞” denotes significant increase from first timepoint (0–10 km) (p < 0.01), “-” denotes significant increase from second timepoint (10–20 km) (p < 0.05), “ = ” denotes significant decrease from third timepoint (20–30 km) (p < 0.05), “#” denotes significant decrease from first timepoint (0–10 km) (p < 0.001), “^” denotes significant decrease from second timepoint (10–20 km) (p < 0.01), and + denotes significant increase from third timepoint (20–30 km) (p < 0.05). “*” denotes a significant condition difference throughout all timepoints (p < 0.01)
Acid-base balance
Blood acid–base balance (blood HCO3− and blood pH) at baseline was similar between conditions (blood HCO3−, MD = 0.34 mmol L−1, p = 0.082; blood pH, MD = 0.002 pH units, p = 0.83). The ingestion of NaHCO3 significantly increased blood HCO3− and blood pH in comparison to the placebo (f = 84.82, p < 0.001, pη2 = 0.87, f = 91.04, p < 0.001, pη2 = 0.88, respectively). Both of these blood variables increased beyond baseline measurements following ingestion of NaHCO3 at pre-exercise (blood HCO3−, MD = 5.6 mmol L−1, p < 0.001; blood pH, MD = 0.052 pH units, p = 0.001) and remained elevated at post warm-up (blood HCO3−, MD = 4.27 mmol L−1, p < 0.001; blood pH, MD = 0.037 pH units, p = 0.01). From baseline measurements to the end of the 40 km TT, blood HCO3− and blood pH changed considerably (f = 28.28, p < 0.001, pη2 = 0.69, f = 5.84, p < 0.001, pη2 = 0.31, respectively). Between NaHCO3 and placebo conditions, both blood metabolites followed comparable patterns during each 40 km TT, each increasing from 10 to 30 km, followed by a subsequent decrease from 30 to 40 km (Fig. 3). This resulted in significant condition-time interaction effects for blood HCO3− (f = 22.74, p < 0.001, pη2 = 0.64) and blood pH (f = 12.10, p < 0.001, pη2 = 0.48).
Mean ± SD blood HCO3− (A) and blood pH (B) responses before and throughout 40 km TT after NaHCO3 and placebo ingestion. “ + ” denotes significant increase from first time point (baseline) (p < 0.01), “∞” denotes significant decrease from first (pre-exercise) and second (post warm-up) time point (p < 0.01), “-” denotes significant decrease from second time point (pre-exercise) (p = 0.01), “ = ” denotes significant increase from fourth time point (10 km) (p < 0.05), “#” denotes significant decrease from sixth time point (30 km) (p < 0.005), “*” denotes a significant condition difference between NaHCO3 and placebo conditions (p < 0.01)
Respiratory responses
The VO2 responses (Fig. 4) did not differ between conditions (f = 0.42, p = 0.52, pη2 = 0.031), but VCO2 was higher following ingestion of NaHCO3 (f = 11.41, p = 0.005, pη2 = 0.47). Consequently, RER was also increased in the NaHCO3 TT’s (f = 12.92, p = 0.003, pη2 = 0.50). Additionally, VO2, VCO2, and RER followed similar patterns during each TT (VO2, f = 7.96, p = 0.006, pη2 = 0.38; VCO2, f = 16.13, p < 0.001, pη2 = 0.73; RER, f = 47.33, p < 0.001, pη2 = 0.50), each decreasing after the opening 9–10 km. Minute ventilation (VE) was unaffected by either method of treatment (condition, f = 0.14, p = 0.71, pη2 = 0.011; distance, f = 3.61, p = 0.059, pη2 = 0.38). There was no condition*distance interaction effect observed for either of the respiratory response measurements (VO2, f = 0.31, p = 0.73, pη2 = 0.024; VCO2, f = 0.17, p = 0.98, pη2 = 0.003; RER, f = 0.59, p = 0.57, pη2 = 0.089).
Mean ± SD VCO2 (A), VO2 (B), RER (C), heart rate (D), blood lactate (E), and RPE (F) responses throughout 40 km TT after NaHCO3 and placebo ingestion. “^” denotes significant decrease from first timepoint (9-10 km) (p < 0.05). “#” denotes significant increase from first timepoint (10 km) (p < 0.001). “+” denotes significant increase from second timepoint (20 km) (p < 0.01). “-” denotes significant increase from third timepoint (30 km) (p < 0.01). “*” denotes significant condition difference at all timepoints (p < 0.01)
Heart rate, blood lactate, RPE, and cadence
Despite the present study revealing a performance difference, both heart rate and RPE were similar across both TTs (Fig. 4; heart rate, f = 0.98, p = 0.34, pη2 = 0.070; RPE, f = 0.59, p = 0.46, pη2 = 0.043). Additionally, cadence was unaffected by either condition (f = 0.99, p = 0.35, pη2 = 0.069) or distance (f = 0.345, p = 0.168, pη2 = 0.35). Conversely, blood lactate was higher throughout each 10 km split in the NaHCO3 trial compared to the placebo (f = 18.35, p < 0.001, pη2 = 0.59,). Heart rate and RPE each increased over the course of the TT (heart rate, f = 24.27, p < 0.001, pη2 = 0.87; RPE, f = 104.17, p < 0.001, pη2 = 0.97), whereas blood lactate maintained a similar pattern over the course of the TT, with responses being greater at 40 km in comparison to every other timepoint (f = 6.98, p = 0.005, pη2 = 0.35). This subsequently led to no condition*distance interaction effect between either of these measures (cadence, f = 0.31, p = 0.82, pη2 = 0.078; heart rate, f = 0.98, p = 0.34, pη2 = 0.070; RPE, f = 0.51, p = 0.68, pη2 = 0.122; blood lactate, f = 0.25, p = 0.89, pη2 = 0.65).
Electrolyte analysis
Blood [Na+] following NaHCO3 supplementation was increased throughout the TTs (Table 1; f = 112.75, p < 0.001, pη2 = 0.90). In contrast, throughout the NaHCO3 TTs, blood [K+] was reduced in comparison to the placebo TTs (f = 17.87, p < 0.001, pη2 = 0.58). Likewise, following NaHCO3 supplementation, both blood [Ca2+] (f = 109.69, p < 0.001, pη2 = 0.89, and blood [Cl−] (f = 18.43 p < 0.001, pη2 = 0.59) decreased in comparison to the placebo TTs. Only blood [Ca2+] followed a similar pattern during the TTs throughout both conditions (f = 3.09, p < 0.001, pη2 = 0.19). There was no condition*distance interaction effect for [K+] (f = 1.59, p = 0.25, pη2 = 0.30), [Ca2+] (f = 0.52, p = 0.68, pη2 = 0.04), [Cl−] (f = 0.50, p = 0.69, pη2 = 0.57), or [Na+] (f = 1.46, p = 0.69, pη2 = 0.29) (Table 1).
Gastrointestinal responses
Prior to exercise in both experimental conditions, no participant experienced any GIS. Additionally, the present study revealed no significant differences between either condition for total GIS after ingestion and prior to exercise (NaHCO3, 22 AU; Placebo, 44 AU; z = − 1.71, p = 0.088, r = 0.46), post exercise (NaHCO3, 76 AU; Placebo, 63 AU; z =− 0.51, p = 0.61 r = 0.14) or for total aggregated GIS (NaHCO3, 98 AU; Placebo, 107 AU; z = − 1.45, p = 0.15, r = 0.39). Peak GIS was also not influenced at either stage (Table 2; pre-exercise, z = − 1.47, p = 0.14, r = 0.39; post-exercise, z = − 0.04, p = 0.97, r = 0.011).
Discussion
The primary aim of the present study was to investigate the effects of NaHCO3 ingestion on 40 km TT cycling performance in trained male cyclists. This is the first study to observe, that ingestion of 0.3 g kg−1 of NaHCO3 supplementation in the form of mini-tablets in a CHO hydrogel (Maurten Bicarb System) improves 40 km TT cycling performance by 1.42%. This represents a small, but practically important performance change that is larger than the variability for TT performances in trained cyclists (Laursen et al. 2003; Palmer et al. 1996; Smith et al. 2001), which has been established as being less than 1% (Currell and Jeukendrup 2008). In addition to these ergogenic effects, the present study reported no differences in GIS, HR or VO2 between the NaHCO3 ingestion and that observed in the placebo trials, despite increased performance in the NaHCO3 trial. Given the widespread evidence and research showing that NaHCO3 is well-known for producing GIS through traditional ingestion methods (Hilton et al. 2019), these findings suggest that this novel form of ingestion can serve as a practical ergogenic aid to enhance prolonged high-intensity exercise performance in trained male cyclists.
Currently, less research has investigated NaHCO3 supplementation on prolonged high-intensity exercise performance that extends beyond 10 min in duration. Despite this, in the few studies that have used NaHCO3 ingestion for prolonged high-intensity TT exercise performance, together with the present findings, its ergogenic potential for TT’s appears to extend beyond the perception that it is only useful for short duration exercise. George and MacLaren (1988) found a 17% improvement in TTE with a mean improvement of 263 s, whereas McNaughton et al. (1999) saw total work increase by 13.3% during a 60 min effort. This too, is similar to the improvements in total work at 110% IAT observed by Egger et al., (2014) following NaHCO3 supplementation. In contrast, despite a significant increase of metabolic alkalosis by means of increases in blood pH and HCO3−, cycling for ~ 30 min at 80% VO2peak (Stephens et al. 2002) and running at 110% IAT until exhaustion (Freis et al. 2017), were previously shown to be unaffected by NaHCO3 supplementation. The lack of performance improving effects following NaHCO3 supplementation on prolonged high intensity exercise in these studies could be attributed to the adverse GIS experienced in participants (Freis et al. 2017), or due to exercise performance protocols with poorer reliability. Indeed, most of the work on NaHCO3 and prolonged high intensity exercise has commonly utilised TTE as a measurement of performance along with either NaHCO3 ingestion in fluid or gelatin capsules. However, measurement by means of TTs have been found to have a lower coefficient of variation (CV) in comparison to TTE, which is observed as improved reproducibility (Palmer et al. 1996). This may in part explain why the present study and Leach et al., (2023) were able to observe improvements in more prolonged cycling TT performance following the ingestion of NaHCO3 than in many other previous studies.
The improvement in 40 km TT performance in the present study was observed following the ingestion of NaHCO3 at an individualised timepoint, coinciding the start of exercise with peak alkalosis (Miller et al. 2016). There are comparatively fewer studies that have implemented individualised NaHCO3 ingestion strategies exploring high-intensity exercise longer than 10 min (Lassen et al. 2021; Leach et al. 2023). Interestingly, work by Carr et al. (2011) suggests, to evoke an ergogenic response following NaHCO3 supplementation, a 5 or 6 mmol L−1 increase in blood HCO3− is necessary. This, however, differs from the work of Leach et al. (2023), whereby enterically coated NaHCO3 improved 16.1 km TT performance by 2.5%, despite only producing a mean 3.7 mmol L−1 increase in blood HCO3−, but such ingestion strategies have previously been shown to have lower peak HCO3− responses than traditional methods of ingestion (Hilton et al. 2019). Additionally, Gough et al. (2022) reported an increase of 2.8 mmol L−1 in blood HCO3− compared to a placebo, which enhanced 4 km cycling TT performance by 1.6% in hot (30 °C) conditions. Both studies are below the previously acclaimed theoretical metabolic threshold to enhance performance. Interestingly, the mean increase in HCO3− that Leach et al. (2023) observed in gelatin encapsulation (5.7 mmol L−1) is similar to the present study’s pre-exercise HCO3− of 5.6 mmol L−1. What is clear, is that the acid–base response between ingestion methods is different. The present study optimised ingestion time, observed a large change in blood HCO3−, and found little or no GIS. These factors may therefore maximise the potential of observing performance improvements but given the likely HCO3− responses following NaHCO3 using this ingestion method (Gough and Sparks 2024), it is not clear if an individualised ingestion time is required. Further research is therefore required to ascertain the effectiveness of NaHCO3 on ecologically valid, high-intensity exercise performance tasks, using a wide variety of ingestion types and timing methods.
In the current study, blood metabolites, such as HCO3−, pH, and lactate were elevated to a greater extent throughout the TT following NaHCO3 supplementation. Following NaHCO3 supplementation, during prolonged high-intensity exercise, a higher efflux of lactate has been observed (Hollidge-Horvat et al. 2000; Siegler et al. 2016; Egger et al. 2014). Work by Hollidge-Horvat et al. (2000) suggests that exercise-induced metabolic alkalosis may increase the rate of glycolysis, resulting in a difference in pyruvate production leading to elevated lactate concentrations. Increased blood lactate concentrations have also been shown to result from improved lactate efflux following alkalosis, even without alterations to skeletal muscle function (Spriet et al. 1986). Intriguingly, improved performance times and power output occurred in the present study, without changes to cadence, heart rate, RPE, or VO2, suggesting increased torque and efficiency, which was not previously observed in 2 km TT performance (Voskamp et al. 2020). The observed increases in VCO2 throughout the 40 km TT following NaHCO3 supplementation may also suggest increased glycolytic rate, increased H+ efflux and buffering (Hirakoba et al. 1993; Requena et al. 2005), or both given the differences in power output. This is however contradictory to previous meta-analytical data deeming NaHCO3 inadequate to enhance VCO2 production throughout aerobic exercise (Calvo et al. 2021), but this may be explained by the diverse study methods, participants and ingestion protocols included in their analysis. Nonetheless RER was elevated in present study, an observation which was also made by Egger et al., (2014). Clearly, further research is required to investigate these responses following the use of the present NaHCO3 ingestion strategy.
The current study also observed decreases in [K+] throughout the TT following NaHCO3 supplementation. Previous research has suggested a potential association between the reduction of H+ in the interstitium following induced metabolic alkalosis with a subsequent decrease in [K+]. This may provide a potential protective function against the reduction in muscle force and fatigue, induced by [K+] (Douroudos et al. 2006). The increased extracellular buffering capacity explained by increases in HCO3− and blood pH, in addition to the increases in blood lactate, and decreases in [K+], likely further explain the improvements in 40 km TT performance derived by NaHCO3 supplementation and warrant further investigation in prolonged exercise.
Ingestion of NaHCO3 is renowned for causing GIS (Carr et al. 2011; Kahle et al. 2013; McNaughton et al. 2019), whereby previous NaHCO3 research has observed ergolytic effects (Cameron et al. 2010), with some research only reporting significant performance improvements when participants with GIS were excluded from the statistical analyses (Saunders et al. 2014). The results of the present study align with recent research suggesting that the delivery method of mini-tablets in CHO hydrogel reduces GIS in comparison to traditional delivery methods of GIS, such as encapsulation (Gough and Sparks 2024). In the present study, six of the participants reported no GIS after NaHCO3 supplementation and those that did, reported only minor to mild GIS responses. Therefore reducing, or in some cases, eliminating, the GIS response has the potential to enhance the ergogenic capacity of this ergogenic aid. Interestingly, all participants reported some GIS in the placebo trial, but this may have been due to the presence of isomalt, and the performance of high intensity prolonged cycling. Further work is therefore required to compare the GIS responses following ingestion of the Maurten Bicarb System to other placebos.
Future directions and limitations
The present study had limitations that should be acknowledged. The results are specific to trained male cyclists, so using the present data to make inferences to other populations should be done with caution. Importantly, sex-based differences are possible (Carr et al. 2023), given that female biological differences such as lower muscle mass and number of type II muscle fibres, may lead to decreased whole body glycolytic rate, potentially resulting in lower H+ accumulation (Hegge et al. 2016; Janssen et al. 2000; Russ et al. 2005). The inclusion of an additional control and NaHCO3 trial would have been useful to specifically determine the test–retest reliability in the present participant population and to verify the performance improvements. However, researchers must clearly balance what might be ideal, with the practicalities of actually being able to recruit enough participants and being able to have them complete all trials in a short enough period that training status changes do not add an additional variable into the study. Consequently, based on experience of running many similar studies, the present design was chosen.
The observed respiratory responses were unexpected, consequently, gas samples were only obtained at three time points during in the TT’s. Future work should therefore attempt to shed more light on the potential impact of NaHCO3 on efficiency during exercise or by specifically conducting economy assessment protocols. The present study only investigated one dose of NaHCO3, similar to that recommended by the manufacturer. However, it is not clear if different doses of NaHCO3 are as or more effective than 0.3 g kg−1 using this delivery system. With such a method of ingestion that limits GIS, future research should therefore consider a dose response study design. Future research should also investigate the use of this NaHCO3 ingestion strategy in participants with a different training status and in females.
Conclusion
The present study is the first to investigate 40 km TT cycling performance following an individualised ingestion protocol of NaHCO3 in the form of mini-tablets in a CHO hydrogel. The study demonstrates that ingestion of 0.3 g kg−1 NaHCO3 in this form can enhance 40 km TT performance by 1.42% and causes minimal GIS in a trained male cyclist population. The performance enhancement in 40 km TT cycling performance following NaHCO3 ingestion is likely due to an increased blood buffering capacity, with reduced relative oxygen cost suggesting improved gross efficiency. The present study demonstrates a large blood HCO3− response with low GIS, which was similar to the GIS of a low sodium containing placebo, can improve 40 km TT cycling performance. Athletes, sports nutritionists, and practitioners should therefore consider the potential for this method of NaHCO3 ingestion to be ergogenic for prolonged high-intensity exercise.
Data availability
The data that support the findings of this study are available from the corresponding author, ESS, upon request.
Abbreviations
- ANOVA:
-
Analysis of Variance
- Ca2+ :
-
Calcium
- CHO:
-
Carbohydrate
- Cl− :
-
Chloride
- CO2 :
-
Carbon dioxide production
- CV:
-
Coefficient of variation
- GIS:
-
Gastrointestinal symptoms
- H+ :
-
Hydrogen ion
- HCO3 − :
-
Bicarbonate anion
- IAT:
-
Individual anaerobic threshold
- K+ :
-
Potassium
- Na+ :
-
Sodium
- NaHCO3 :
-
Sodium bicarbonate
- RER:
-
Respiratory exchange ratio
- RPE:
-
Rating of perceived exertion
- TT:
-
Time trial
- TTE:
-
Time to exhaustion
- TTP:
-
Time to peak
- VO2 :
-
Oxygen uptake
- VO2Peak :
-
Peak oxygen uptake
References
Boegman S, Stellingwerff T, Shaw G, Clarke N, Graham K, Cross R, Siegler JC (2020) The impact of individualizing sodium bicarbonate supplementation strategies on world-class rowing performance. Front Nutr 9(7):138. https://doi.org/10.3389/fnut.2020.00138
Borg G (1982) Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int J Sports Med 3(03):153–158. https://doi.org/10.1055/s-2008-1026080
Calvo J, Xu H, Mon-López D, Pareja-Galeano H, Jiménez S (2021) Effect of sodium bicarbonate contribution on energy metabolism during exercise: a systematic review and meta-analysis. J Int Soc Sports Nutr 18(1):11. https://doi.org/10.1186/s12970-021-00410-y
Cameron SL, McLay-Cooke RT, Brown RC, Gray AR, Fairbairn KA (2010) Increased blood pH but not performance with sodium bicarbonate supplementation in elite rugby union players. Int J Sport Nutr Exerc Metab 20(4):307–321. https://doi.org/10.1123/ijsnem.20.4.307
Carr A, Slater G, Gore C, Dawson B, Burke L (2011) Effect of sodium bicarbonate on [HCO3−], pH, and gastrointestinal symptoms. Int J Sport Nutr Exerc Metab 21(3):189–194. https://doi.org/10.1123/ijsnem.21.3.189
Carr AJ, McKay AK, Burke LM, Smith ES, Urwin CS, Convit L, Jardine WT, Kelly MK, Saunders B (2023) Use of buffers in specific contexts: highly trained female athletes, extreme environments and combined buffering agents—a narrative review. Sports Med 53(Suppl 1):25–48. https://doi.org/10.1007/s40279-023-01872-7
Cohen J (1988) Statistical power analysis for the behavioural sciences, 2nd edn. Lawrence Erlbaum Associates, Hillsdale
Currell K, Jeukendrup AE (2008) Validity, reliability and sensitivity of measures of sporting performance. Sports Med 38:297–316. https://doi.org/10.2165/00007256-200838040-00003
de Oliveira LF, Dolan E, Swinton PA, Durkalec-Michalski K, Artioli GG, McNaughton LR, Saunders B (2021) Extracellular buffering supplements to improve exercise capacity and performance: a comprehensive systematic review and meta-analysis. Sports Med. https://doi.org/10.1007/s40279-021-01575-x
Deb S, Gough L, Sparks S, McNaughton L (2018) Sodium bicarbonate supplementation improves severe-intensity intermittent exercise under moderate acute hypoxic conditions. Eur J Appl Physiol 118(3):607–615. https://doi.org/10.1007/s00421-018-3801-7
Douroudos II, Fatouros IG, Gourgoulis V, Jamurtas AZ, Tsitsios T, Hatzinikolaou A, Margonis K, Mavromatidis K, Taxildaris K (2006) Dose-related effects of prolonged NaHCO3 ingestion during high-intensity exercise. Med Sci Sports Exerc 38(10):1746–1753. https://doi.org/10.1249/01.mss.0000230210.60957.67
Egger F, Meyer T, Such U, Hecksteden A (2014) Effects of sodium bicarbonate on high intensity endurance performance in cyclists: a double-blind, randomized cross-over trial. PLoS ONE 9(12):e114729. https://doi.org/10.1371/journal.pone.0114729
Folland JP, Stern R, Brickley G (2008) Sodium phosphate loading improves laboratory cycling time-trial performance in trained cyclists. J Sci Med Sport 11(5):464–468. https://doi.org/10.1016/j.jsams.2007.04.004
Freis T, Hecksteden A, Such U, Meyer T (2017) Effect of sodium bicarbonate on prolonged running performance: a randomized, double-blind, cross-over study. PLoS ONE 12(8):e0182158. https://doi.org/10.1371/journal.pone.0182158
George K, MacLaren D (1988) The effect of induced alkalosis and acidosis on endurance running at an intensity corresponding to 4 mM blood lactate. Ergonomics 31(11):1639–1645. https://doi.org/10.1080/00140138808966813
Gough L, Sparks SA (2024) The effects of a carbohydrate hydrogel system for the delivery of bicarbonate mini-tablets on acid-base buffering and gastrointestinal symptoms in resting well-trained male cyclists. Sports Med Open. https://doi.org/10.1186/s40798-024-00684-x
Gough L, Deb S, Sparks A, McNaughton L (2017) The reproducibility of blood acid base responses in male collegiate athletes following individualised doses of sodium bicarbonate: a randomised controlled crossover study. Sports Med 47(10):2117–2127. https://doi.org/10.1007/s40279-017-0699-x
Gough LA, Williams JJ, Newbury JW, Gurton WH (2022) The effects of sodium bicarbonate supplementation at individual time-to-peak blood bicarbonate on 4-km cycling time trial performance in the heat. Eur J Sport Sci 22(12):1856–1864. https://doi.org/10.1080/17461391.2021.1998644
Gough L, Newbury J, Price M (2023) The effects of sodium bicarbonate ingestion on swimming interval performance in trained competitive swimmers. Eur J App Physiol. https://doi.org/10.1007/s00421-023-05192-6
Hegge AM, Bucher E, Ettema G, Faude O, Holmberg HC, Sandbakk Ø (2016) Gender differences in power production, energetic capacity and efficiency of elite cross-country skiers during whole-body, upper-body, and arm poling. Eur J Appl Physiol 116:291–300. https://doi.org/10.1007/s00421-015-3281-y
Hilton NP, Leach NK, Sparks SA, Gough LA, Craig MM, Deb SK, McNaughton LR (2019) A novel ingestion strategy for sodium bicarbonate supplementation in a delayed release form: a randomised crossover study in trained males. Sports Med-Open 5(1):1–8. https://doi.org/10.1186/s40798-019-0177-0
Hilton NP, Leach NK, Hilton MM, Sparks SA, McNaughton LR (2020) Enteric coated Sodium bicarbonate supplementation improves high intensity cycling performance in trained cyclists. Eur J Appl Physiol 120(7):1563–1573. https://doi.org/10.1007/s00421-020-04387-5
Hirakoba K, Maruyama A, Misaka K (1993) Effect of acute sodium bicarbonate ingestion on excess CO2 output during incremental exercise. Eur J Appl Physiol 66:536–541. https://doi.org/10.1007/bf00634306
Hollidge-Horvat M, Parolin M, Wong D, Jones N, Heigenhauser G (2000) Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Am J Physiol Endocrinol Metab 278(2):316–329. https://doi.org/10.1152/ajpendo.2000.278.2.e316
Ivarsson A, Andersen MB, Johnson U, Lindwall M (2013) To adjust or not adjust: nonparametric effect sizes, confidence intervals, and real-world meaning. Psychol Sport Exerc 14(1):97–102. https://doi.org/10.1016/j.psychsport.2012.07.007
Janssen I, Heymsfield SB, Wang Z, Ross R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. https://doi.org/10.1152/jappl.2000.89.1.81
Kahle LE, Kelly PV, Eliot KA, Weiss EP (2013) Acute sodium bicarbonate loading has negligible effects on resting and exercise blood pressure but causes gastrointestinal distress. Nutr Res 33(6):479–486. https://doi.org/10.1016/j.nutres.2013.04.009
Lassen TA, Lindstrøm L, Lønbro S, Madsen K (2021) Increased performance in elite runners following individualized timing of sodium bicarbonate supplementation. Int J Sport Nutr Exerc Metab 31(6):453–459. https://doi.org/10.1123/ijsnem.2020-0352
Laursen PB, Shing CM, Jenkins DG (2003) Reproducibility of a laboratory-based 40-km cycle time-trial on a stationary wind-trainer in highly trained cyclists. Int J Sports Med 24(07):481–485. https://doi.org/10.1055/s-2003-42012
Leach NK, Hilton NP, Tinnion D, Dobson B, McNaughton LR, Sparks SA (2023) Sodium bicarbonate ingestion in a fasted state improves 16.1 km cycling time trial performance. Med Sci Sports Exerc 55(12):2299–2307. https://doi.org/10.1249/mss.0000000000003263
Martin R, Hilton N, Sparks S, Saunders B, McNaughton L (2023) The effects of enteric-coated sodium bicarbonate supplementation on 2 km rowing performance in female CrossFit® athletes. Eur J App Physiol 123(6):1191–1198. https://doi.org/10.1007/s00421-023-05140-4
McKay AK, Stellingwerff T, Smith ES, Martin DT, Mujika I, Goosey-Tolfrey VL, Sheppard J, Burke LM (2021) Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform 17(2):317–331. https://doi.org/10.1123/ijspp.2021-0451
McNaughton LR (1992) Sodium bicarbonate ingestion and its effects on anaerobic exercise of various durations. J Sports Sci 10(5):425–435. https://doi.org/10.1080/02640419208729941
McNaughton L (2019) A novel ingestion strategy for sodium bicarbonate supplementation in a delayed release form: a randomised crossover study in trained males. Sports Med Open 5(1):1–8. https://doi.org/10.1186/s40798-019-0177-0
McNaughton L, Dalton B, Palmer G (1999) Sodium bicarbonate can be used as an ergogenic aid in high-intensity, competitive cycle ergometry of 1 h duration. Eur J Appl Physiol 80(1):64–69. https://doi.org/10.1007/s004210050559
McNaughton L, Gough L, Deb S, Bentley D, Sparks SA (2016) Recent developments in the use of sodium bicarbonate as an ergogenic aid. Curr Sports Med Rep 15(4):233–244. https://doi.org/10.1249/jsr.0000000000000283
McNaughton L, Brewer C, Deb S, Hilton N, Gough L, Sparks A (2019) Buffering agents: Sodium bicarbonate, sodium citrate and sodium phosphate. Dietary Supplementation in Sport and Exercise. Routledge, pp 191–206
Midgley AW, McNaughton LR, Polman R, Marchant D (2007) Criteria for determination of maximal oxygen uptake: a brief critique and recommendations for future research. Sports Med 37:1019–1028. https://doi.org/10.2165/00007256-200737120-00002
Miller P, Robinson A, Sparks S, Bridge C, Bentley D, McNaughton L (2016) The effects of novel ingestion of sodium bicarbonate on repeated sprint ability. J Strength Cond Res 30(2):561–568. https://doi.org/10.1519/jsc.0000000000001126
Morris DM, Shafer RS, Fairbrother KR, Woodall MW (2011) Effects of lactate consumption on blood bicarbonate levels and performance during high-intensity exercise. Int J Sport Nutr Exerc Metab 21(4):311–317. https://doi.org/10.1123/ijsnem.21.4.311
Ööpik V, Saaremets I, Timpmann S, Medijainen L, Karelson K (2004) Effects of acute ingestion of sodium citrate on metabolism and 5-km running performance: a field study. Can J Appl Physiol 29(6):691–703. https://doi.org/10.1139/h04-044
Palmer GS, Dennis SC, Noakes TD, Hawley JA (1996) Assessment of the reproducibility of performance testing on an air-braked cycle ergometer. Int J Sports Med 17(04):293–298. https://doi.org/10.1055/s-2007-972849
Potteiger J, Webster M, Nickel G, Haub M, Palmer R (1996) The effects of buffer ingestion on metabolic factors related to distance running performance. Eur J Appl Physiol 72:365–371. https://doi.org/10.1007/bf00599698
Reilly T (1990) Human circadian rhythms and exercise. Crit Rev Biomed Eng 18(3):165–180
Requena B, Zabala M, Padial P, Feriche B (2005) Sodium bicarbonate and sodium citrate: ergogenic aids? J Strength Cond Res 19(1):213–224. https://doi.org/10.1519/00124278-200502000-00036
Russ DW, Lanza IR, Rothman D, Kent-Braun JA (2005) Sex differences in glycolysis during brief, intense isometric contractions. Muscle Nerve 32(5):647–655. https://doi.org/10.1002/mus.20396
Saunders B, Sale C, Harris RC, Sunderland C (2014) Sodium bicarbonate and high-intensity-cycling capacity: variability in responses. Int J Sports Physiol Perform 9(4):627–632. https://doi.org/10.1123/ijspp.2013-0295
Siegler JC, Marshall PW, Bishop D, Shaw G, Green S (2016) Mechanistic insights into the efficacy of sodium bicarbonate supplementation to improve athletic performance. Sports Med-Open 2(1):1–13. https://doi.org/10.1186/s40798-016-0065-9
Smith MF, Davison RCR, Balmer J, Bird SR (2001) Reliability of mean power recorded during indoor and outdoor self-paced 40 km cycling time-trials. Int J Sports Med 22(04):270–274. https://doi.org/10.1055/s-2001-13813
Sparks A, Williams E, Robinson A, Miller P, Bentley DJ, Bridge C, Mc Naughton LR (2017) Sodium bicarbonate ingestion and individual variability in time-to-peak pH. Res Sports Med 25(1):58–66. https://doi.org/10.1080/15438627.2016.1258645
Spriet LL, Lindinger MI, Heigenhauser GJ, Jones NL (1986) Effects of alkalosis on skeletal muscle metabolism and performance during exercise. Am J Physiol 251(5):R833–R839. https://doi.org/10.1152/ajpregu.1986.251.5.R833
Stadlbauer V, Wallner S, Stojakovic T, Smolle KH (2011) Comparison of 3 different multianalyte point-of-care devices during clinical routine on a medical intensive care unit. J Crit Care 26(4):433-e1. https://doi.org/10.1016/j.jcrc.2010.09.003
Stephens T, McKenna M, Canny BJ, Snow R, McConnell G (2002) Effect of sodium bicarbonate on muscle metabolism during intense endurance cycling. Med Sci Sports Exerc 34(4):614–621. https://doi.org/10.1097/00005768-200204000-00009
Voskamp AE, van den Bos S, Foster C, de Koning JJ, Noordhof DA (2020) The effect of sodium bicarbonate supplementation on the decline in gross efficiency during a 2000-m cycling time trial. Int J Sports Physiol Perform 15(5):741–747. https://doi.org/10.1123/ijspp.2019-0177
Acknowledgements
The authors would like to thank all of the participants and Dr Joshua Rowe from Maurten AB, Sweden for providing the sodium bicarbonate products.
Author information
Authors and Affiliations
Contributions
Study conceptualisation was conducted by ESS, SAS and LRM. ESS, AR, BD and XY collected the data. Data were analysed by ESS. The manuscript was principally written by ESS, with contributions from SAS and LRM. All authors read each draft of the manuscript, provided feedback and approved the final version.
Corresponding author
Ethics declarations
Conflicts of interest
ESS, AR, BD, XY, and LRM have no conflicts of interest. At the time of study design, data collection and analysis, SAS was a colleague of ESS, AR, BD, and LRM, but is now employed by Maurten AB, Sweden. Maurten AB Sweden had no input to the design, data collection, analysis, or interpretation of these data. Maurten AB provided the sodium bicarbonate product free of charge.
Additional information
Communicated by Michael I Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shannon, E.S., Regnier, A., Dobson, B. et al. The effect of sodium bicarbonate mini-tablets ingested in a carbohydrate hydrogel system on 40 km cycling time trial performance and metabolism in trained male cyclists. Eur J Appl Physiol (2024). https://doi.org/10.1007/s00421-024-05567-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00421-024-05567-3