Abstract
Purpose
The purpose of this study was to investigate the acute effect of head impacts, sustained over the course of three rounds of amateur boxing, on indices of cerebrovascular function.
Methods
Eighteen university amateur boxers (six female) completed three experimental trials in a randomised order; (1) three rounds of boxing (BOX), (2) an equivalent bout of pad boxing (where no blows to the head were sustained; PAD), and (3) a time-matched seated control trial (CON). Indices of cerebrovascular function were determined immediately before and 45 min after each trial. Specifically, dynamic cerebral autoregulation (dCA) was determined by considering the relationship between changes in cerebral blood velocity and mean arterial pressure during 5 min of squat-stand manoeuvres at 0.05 and 0.10 Hz. Cerebrovascular reactivity was determined using serial breath holding and hyperventilation attempts.
Results
Participants received an average of 40 ± 16 punches to the head during the BOX trial. Diastolic, mean and systolic dCA phase during squat stand manoeuvres at 0.05 Hz was lower after BOX compared to pre BOX (P ≤ 0.02, effect size (d) ≥ 0.74). No other alterations in dCA outcomes were observed at 0.05 or 0.10 Hz. The number of head impacts received during the BOX trial was associated with the change in systolic phase (r = 0.50, P = 0.03). No differences in cerebrovascular reactivity to breath holding or hyperventilation were observed.
Conclusions
A typical bout of amateur boxing (i.e., three rounds) can subtly alter cerebral pressure-flow dynamics, and the magnitude of this change may be related to head impact exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the pluripotent benefits of sports participation, there is concern that individuals engaged in contact sports may be at an elevated risk of neurodegenerative disease and dementia (Lehman et al. 2012; Mackay et al. 2019). It has recently been argued that the repeated exposure to sub-concussive head impacts might explain at least some of this risk (Russell et al. 2021; Nowinski et al. 2022). Given the devastating nature of such diseases, and that no curative treatment is currently available for dementia, identifying the underlying pathophysiological pathways associated with repetitive head impacts is recognised as a contemporary, public health priority (Livingston et al. 2020).
The brain blood flow response to alterations in blood pressure (cerebral autoregulation; CA) and carbon dioxide (cerebrovascular reactivity; CVR–CO2) reflect critical regulatory processes. A growing body of evidence indicates that these responses may be altered in contact sport athletes (Bailey et al. 2013; Wright et al. 2018a; Marley et al. 2021; Owens et al. 2021). Bailey et al. (2013), identified that professional boxers presented with altered CA response to a sit-to-stand transition and a thigh cuff occlusion challenge, and impaired CVR–CO2 to hypercapnia (5% CO2 inhalation) and hypocapnia (hyperventilation) compared to their age, sex and fitness-matched peers. Importantly, the impairment in CVR–CO2 observed was only associated with the volume and intensity of sparring during training (“sparring index”) rather than frequency of total knockouts experienced over the course of a career, which tallies with the prevailing concern regarding the accumulation of sub-concussive impacts.
It is possible that these chronic changes in CA and CVR–CO2 are driven by the repeated, acute alterations following exposure to head impacts (Huber et al. 2016). Therefore, it is important to understand whether the regulation of brain blood flow can be acutely altered by head impacts. Recent evidence demonstrates that heading in football may acutely alter dynamic CA (dCA) during repeated squat-stand transitions (Smirl et al. 2022) and other metrics of cerebral blood flow regulation (Smirl et al. 2020). However, our own work, which utilised fewer headers, did not identify any acute changes in dCA during repeated squat-stand manoeuvres, nor CVR–CO2 to repeated breath holding or hyperventilation challenges (Jack et al. 2022). Therefore, a dose–response relationship might exist regarding the number of subconcussive head impacts, and acute alterations in brain blood flow regulation. For example, changes in dCA metrics after a season of contact sports are associated with the frequency of head impact exposures (Wright et al. 2018a). However, few experimental data are available in this emerging field.
As the primary aim of boxing is to deliver repetitive blows to the head to score points, or win by knock-out, the sport offers a unique model to observe the effects of repetitive head impacts. The purpose of this study was to characterise any changes in dCA and CVR–CO2 following a typical amateur boxing match. A secondary aim was to consider whether any such changes were related to the acute cumulative head impact exposure. We hypothesised that acute alterations in dCA would be apparent post boxing, and that these changes would be positively associated with the number of head impacts experienced.
Methods
Participants
Following institutional ethical approval, 18 university amateur boxers (6 female; age 21 ± 1 y; mass 76.5 ± 14.6 kg; stature 1.74 ± 0.09 m; body mass index 25.0 ± 2.3 kg/m2) provided written informed consent to take part in the study. Using our unpublished pilot data, we calculated a priori that 17 participants would be needed to detect a time by trial interaction effect for dCA (phase—as determined by transfer function analysis) in a repeated, within-measures design. Participants had 3 ± 2 years of boxing experience, were active members of the competitive university squad, and took part in at least 3 rounds of sparring per week. The number of concussions previously sustained were as follows: 0 concussions n = 13, 1 concussion n = 3, 2 concussions n = 1 and 3 concussions n = 1. None of these concussions occurred within the 3 months prior to our study. All participants had passed a medical examination and were members of England Boxing (formally the Amateur Boxing Association of England). Exclusion criteria included any contraindications to exercise, history of drug abuse, smoking and the use of any medication which could influence blood pressure, heart rate, or vascular function.
Experimental design
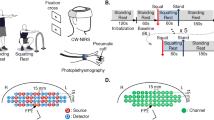
Participants were paired according to sex and mass, to prevent physical mismatches. Following a preliminary visit to the laboratory for familiarisation of all procedures and collection of descriptive data, each pair completed three experimental trials in a randomised order; boxing (BOX), pad-boxing (PAD), and a seated control trial (CON). Each visit occurred at the same time of day, and were separated by approximately 1 week. Participants were instructed to arrive at the laboratory having avoided vigorous exercise (Burma et al. 2020b) and caffeine (Peng et al. 2022) in the 24 and 4 h prior, respectively.
The BOX trial consisted of three separate rounds, each 3 min in duration, separated by 1 min of rest, as specified by the English amateur boxing rulebook (England Boxing 2022). Participants all wore regulation weight boxing gloves (12 oz). Each BOX session was completed at a boxing club, so that it could be overseen by a medically trained boxing coach and video recorded from two separate angles. Participants were driven (~ 10 min) to the club and back again by a member of the research team. The PAD trial was performed in the laboratory, and designed to replicate the exertion of a boxing session but without the exposure to head impacts. Participants completed the same format (three rounds) of punching hand pads held by a member of the boxing club. The CON trial consisted of time-matched seated rest (11 min) in the laboratory. Experimental outcomes were collected immediately before and 45 min after each trial, which provided sufficient time to re-instrument participants and ensure they were at rest prior to re-assessment of dCA outcomes.
Estimation of head impacts
Two researchers reviewed the video footage and independently tallied the number of head impacts received by each boxer. The estimated total number of head impacts sustained across all bouts never differed by more than 5 punches between the two researchers (intraclass correlation coefficient = 0.998, P < 0.01). Thus, an average of these two values was taken for each BOX trial.
Instrumentation
Blood velocity in the middle cerebral artery (MCAv) was measured using transcranial Doppler ultrasonography (DWL, Compumedics, Germany) to provide an estimation of cerebral blood flow (Willie et al. 2011; Ainslie and Hoiland 2014). A 2 MHz probe was positioned over the right trans-temporal window and secured with an adjustable headset (DiaMon, DWL, Germany). The position of the probe and insonation depth were noted and closely replicated within an individual across trials. The between-day coefficient of variation for resting MCAv (taken as the mean baseline value prior to the first BH attempt) was 7.6%. Beat-by-beat mean arterial pressure (MAP) was continuously measured by finger photoplethysmography (Finometer PRO, Netherlands) with correction to account for any height differences between the index finger and heart. R–R intervals were quantified using a 3 lead electrocardiogram. Partial pressure of end-tidal carbon dioxide (PETCO2) was sampled via a mouthpiece and recorded using an online gas analyser (ML206; ADInstruments, USA). A nose clip was applied throughout to ensure inspiration and expiration was performed through the mouthpiece. MCAv, MAP, heart rate and PETCO2 were sampled continuously at 200 Hz and integrated using an analogue-to-digital converter (Powerlab; model—8/30, ADInstruments, USA) linked with a laptop computer. All data were stored for later analysis using commercially accessible software (LabChart v8, ADInstruments, USA).
Cerebral autoregulation
Repeated squat-stand manoeuvres (SSM) were used to assess dCA, in line with current recommendations (Smirl et al. 2015; Panerai et al. 2023). Participants underwent two separate 5-min SSM at 0.05 and 0.10 Hz, achieving a ~ 90° knee-bend with each squat. The order of the two SSM protocols were kept consistent within a participant, but counterbalanced between participants. Participants were given 2 min seated recovery between tests and were instructed to stand still for 1 min prior to initiation of the remaining SSM to allow recovery from initial orthostatic hypotension (Narayanan et al. 2001).
Metrics of dCA were determined for each SSM frequency and quantified using transfer function analysis (Panerai et al. 2023), using dedicated software (Elucimed, Ensemble-R, New Zealand) which incorporated R–R interval calibration. Output summaries of coherence, phase (radians), gain (cm/s/mmHg) and normalised gain (%/%) for 0.05 Hz and 0.10 Hz SSM were calculated at the point estimates of the SSM frequency, verified by visual inspection of blood pressure and MCAV power spectrum densities (Smirl et al. 2015). Due to evidence that dCA may be differentially regulated across the cardiac cycle (Smirl et al. 2018), and to provide insight which might otherwise be missed (Burma et al. 2020b), alterations in dCA metrics were further scrutinised using the systolic and diastolic components of each cardiac cycle. There was no evidence of phase wrap-around at any point estimate. One participant was removed from 0.10 Hz SSM analysis due to a poor MAP signal acquisition which resulted in an abnormally low coherence value (< 0.70) for one visit.
Cerebrovascular reactivity to carbon dioxide
CVR–CO2 during hypercapnia and hypocapnia was measured whilst seated using a repeated breath holding (BH) and hyperventilation (HV) protocol, respectively. This approach has been shown to be sensitive to alterations in CVR–CO2 in the days following concussion (Len et al. 2011). The BH and HV protocols were performed in a counterbalanced order between participants, but consistent within a participant. Baseline MCAv, MAP, cerebrovascular conductance (MCAv/MAP) and cerebrovascular resistance (MAP/MCAv) were measured for 1 min following approximately 5 min of seated rest. Following a normal inspiration, participants were then instructed to hold their breath for 20 s whilst remaining relaxed. Participants were coached to avoid performing the Valsalva manoeuvre during their first familiarisation visit. This was repeated five times in total, interspersed by 40 s recovery. The CVR–CO2 to repeated BH was quantified as the percentage increase in the greatest MCAv value above baseline following each breath hold attempt (BHMCAv%), in line with previous work from our laboratory (Koep et al. 2020, 2021; Jack et al. 2022). To better isolate the reactivity to carbon dioxide and minimise the influence of any changes in MAP or Valsalva manoeuvre on MCAv, individual breath hold attempts were removed from analysis if MAP increased by more than 15 mmHg. Nine such attempts (out of a total of 540) were rejected across the whole study in total. In addition, the percentage change in MCAv was expressed per unit change in PETCO2 after each BH attempt (BHCVR).
A separate 1 min MCAv and PETCO2 baseline period was provided for the HV protocol. Participants then hyperventilated at 36 breaths per minute for 20 s, followed by 40 s of recovery. This was repeated five times in total. HVMCAv% was determined as the percentage decrease in MCAv from baseline after each hyperventilation period. This percentage change in MCAv was also expressed per unit change in PETCO2 after each HV attempt (HVCVR).
Statistical analyses
Separate three (trial) by two (timepoint) repeated measures analysis of variance tests were used to determine changes in dCA metrics and resting haemodynamic outcomes, whilst three (trial) by two (timepoint) by five (attempt) analysis of variance assessments were used to identify any differences in CVR–CO2 outcomes. Fisher’s least significant differences post hoc analyses were used to explore any significant interaction effects. The main and interaction effects are presented using the P value and partial eta squared (ηp2), which was interpreted as small (< 0.06), moderate (0.06 < ηp2 < 0.14) and large (> 0.14). The magnitude of post-hoc pairwise comparisons were explored using standardised effect sizes (Cohen’s d) and interpreted as small (< 0.20), moderate (0.20–0.50) and large (> 0.50) (Cohen 1988). Pearson’s correlation was used to explore any relationship between the number and estimated accumulated force of received head impacts and the change in cerebrovascular outcomes. All statistical analyses were completed using SPSS (IBM Corp, USA, v26.0).
Results
Each participant completed all trials in their entirety. Participants received an average of 40 ± 16 punches to the head during the BOX trial (range 22–68). The club medic did not note any potential concussive events, and no symptoms of concussion were reported by the participants.
Resting haemodynamic data
No significant interaction effects were observed for baseline MCAv (P = 0.11, η2 = 0.12), MAP (P = 0.73, η2 = 0.02), PETCO2 (P = 0.18, η2 = 0.11), cerebrovascular conductance index (P = 0.45, η2 = 0.05) or cerebrovascular resistance index (P = 0.92, η2 < 0.01).
Squat stand manoeuvres at 0.05 Hz
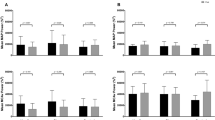
There was no interaction effect for the power spectrum densities for MAP (P = 0.13, η2 = 0.11) or MCAv (P = 0.07, η2 = 0.16) during SSM at 0.05 Hz (Table 1). No trial by time interaction effects were observed for mean coherence (P = 0.98, η2 = 0.01), gain (P = 0.80, η2 = 0.01), or normalised gain (P = 0.78, η2 = 0.02) (Fig. 1). A lack of time by trial interaction was also observed for these dCA metrics during diastole (P > 0.24, η2 < 0.08 for all) and systole (P > 0.25, η2 < 0.08 for all). However, the trial by time interaction for mean phase was P = 0.05, η2 = 0.16. Post-hoc analysis of the interaction revealed a lower mean phase after BOX compared to pre-boxing (0.62 ± 0.17 vs 0.75 ± 0.18 radians, respectively, P = 0.02, d = 0.74). Phase was also significantly lowered after BOX in both systolic (1.00 ± 0.12 vs 1.33 ± 0.12 radians, P = 0.01, d = 2.75,) and diastolic (0.52 ± 0.05 vs 0.65 ± 0.04 radians, P = 0.01, d = 2.87) portions of the cardiac cycle.
Transfer function analysis output from the 0.05 Hz frequency of the squat-stand manoeuvres across all three conditions (CON = seated control trial, PAD = pad boxing (no head impacts) and BOX = boxing). Individual participant data are plotted along with the mean (horizontal line) and standard deviation (error bars). * Indicates a significantly reduced phase after the boxing trial when compared to pre-boxing measures (P = 0.02, d = 0.74)
The change in systolic phase post-boxing (delta phase) was related to the number of head impacts received (r = 0.50, P = 0.03; Fig. 2). This relationship was not observed for the change in diastolic or mean phase post boxing (r < 0.18, P > 0.47). The change in phase across the cardiac cycle post-boxing was never significantly associated with years of boxing experience (r < 0.36, P > 0.15).
Squat stand manoeuvres at 0.10 Hz
There was no time by trial interaction effect for MAP (P = 0.91, η2 = 0.01) or MCAv (P = 0.17, η2 = 0.11) power during SSM at 0.10 Hz. There was no significant interaction effect for mean coherence (P = 0.67 η2 = 0.02), phase (P = 0.90, η2 = 0.01), gain (P = 0.26, η2 = 0.08), or normalised gain (P = 0.89, η2 = 0.01; Fig. 3). Furthermore, no significant interaction effects in dCA metrics were observed in the diastolic (P > 0.10, η2 < 0.13 for all) or systolic (P > 0.13, η2 < 0.12 for all) portions of the cardiac cycle.
Transfer function analysis output from the 0.10 Hz frequency of the squat-stand manoeuvres across all three conditions (CON seated control trial, PAD pad boxing (no head impacts) and BOX boxing). Individual participant data are plotted along with the mean (horizontal line) and standard deviation (error bars). Repeated measures ANOVA revealed no significant interaction effect for any outcome (P > 0.26, η2 < 0.08 for all)
Cerebrovascular reactivity to carbon dioxide
The breath holding protocol typically increased PETCO2 by 3.1 ± 1 mmHg, which was never different (trial by time interaction P = 0.37, η2 = 0.08). The MCAv responses to breath holding was never altered in the study. Specifically, no significant interaction effects were observed for BHMCAv% (P = 0.73, η2 = 0.05; Table 2), and this inference remained when normalising to PETCO2 changes (BHCVR P = 0.72, η2 = 0.03).
Participants typically experienced reductions in PETCO2 of 12.8 ± 2.3 mmHg after each hyperventilation attempt (trial by time interaction P = 0.16, η2 = 0.11). There was no interaction effect for HVMCAv% (P = 0.20, η2 = 0.13; Table 3). No significant differences were observed when expressing this percentage change in MCAv per unit change in PETCO2 (HVCVR P = 0.24, η2 = 0.18).
Discussion
The aim of this study was to identify whether a single bout of amateur boxing acutely alters putatively important metrics of brain blood flow regulation. We observed that many indices of dCA and CVR–CO2 were unchanged following three rounds of boxing; however, there was a delay in the ability to buffer the oscillations in MAP (i.e., phase) when performing SSM at the 0.05 Hz frequency. The absence of such changes in the PAD trial, and the observed association between the change in phase and the number of head impacts received, indicate that subconcussive blows to the head may acutely alter the cerebral pressure–perfusion relationship.
Alterations in the timing offset between changes in MAP and MCAv have previously been observed in male professional boxers (Bailey et al. 2013). Specifically, these authors identified a slower rate of MCAv regulation in boxers using the thigh cuff occlusion technique. Recent interventional work demonstrated that 40 headers, which is similar to the mean number of head impacts received in the present study, augmented (rather than attenuated) phase in young healthy footballers (Smirl et al. 2022). We are unable to reconcile this difference in the direction of the acute change in phase, although it is possible that the nature of the head impact exposure (a soccer heading drill, compared to 3 rounds of boxing) may have a contributory role. For example, greater rotational, but not linear, head accelerations have been reported in collegiate soccer and heading compared to university boxing (Doan et al. 2022), and the consequences of these stimuli are not equal (Zhang et al. 2006; Lota et al. 2022). The physiological challenge of three rounds of boxing (i.e., sympathetic arousal) is also likely to be distinctly different from a heading drill. A decrease in phase has been observed during SSM following a season of contact sports, and the magnitude of this change was also positively associated with the accumulation of head impact accelerations (Wright et al. 2018a). Interestingly, no changes in phase have been observed after 6 headers, despite replicating the study design and dCA methodology (Jack et al. 2022). The correlation between head impacts received and the change in dCA phase observed here suggests that a greater number of headers may have elicited such alterations. Taken together, it appears that alterations in dCA are possible in the acute aftermath of subconcussive head impacts.
The frequency-dependent nature of the subtle change in phase after BOX, where significant differences were observed during SSM at 0.05 Hz but not 0.10 Hz, may indicate alterations in myogenic rather than sympathetic mechanisms of regulation (Hamner et al. 2010; Hamner and Tan 2014). Such findings differ to the changes in phase only observed during 0.10 Hz SSM after concussion (Wright et al. 2018b) and subconcussive impacts (Wright et al. 2018a; Smirl et al. 2022). Collectively, this indicates that autoregulatory changes following head impacts might not be reserved to just one regulatory pathway. Similarly, we observed no changes in dCA gain, which aligns with the response post heading (Smirl et al. 2022). This indicates that perturbations in the speed of the buffering response may occur independently of changes in the ability to dampen the magnitude of the change in MCAv during MAP oscillations. We also report that phase was lower during diastole and systole, although only the latter correlated with the number of head impacts sustained. Differentiating phase in diastole and systole is important given that the oscillations during systole are understood to be more extensively buffered than their pressure-passive diastole counterpart, presumably to provide protection against haemorrhage (Smirl et al. 2018). Future research is needed to explore myogenic and sympathetic regulation mechanisms following head trauma, with acknowledgement of phase during diastole and systole. This work would also benefit from considering the time course of any such changes, as we cannot extrapolate our findings beyond the single measure obtained 45 min post BOX in our study.
The present study found no evidence of alteration in CVR–CO2 following three rounds of amateur boxing. Impairment in CVR has been observed in boxers (Bailey et al. 2013), footballers with a history of heading (Marley et al. 2021) and following a season of professional rugby (Owens et al. 2021). This suggests that a longer time frame of exposure is needed for alterations in CVR–CO2 to manifest; however, none of the aforementioned studies used breath-holding as their hypercapnic challenge. Whilst breath-holding has been advocated as a cerebrovascular stimulus (Tancredi and Hoge 2013; Pinto et al. 2020), our protocol did not provide the same magnitude of increase in PETCO2 which would be expected with traditional CO2 breathing challenges (Koep et al. 2022), nor the same amount of control afforded by end-tidal forcing approaches (Howe et al. 2020; Carr et al. 2021). Such an approach was beyond the scope of this study, and open circuit challenges are not without their issues (Burley et al. 2020). It has been demonstrated that only ~ 65% of the MCAv response to a single breath-hold attempt is related to elevations in arterial CO2 (Przybyłowski et al. 2003). Therefore, our BH protocol is not the same CO2 stimulus as those used by others in this field (Bailey et al. 2013; Marley et al. 2021; Owens et al. 2021), which elicited greater increases in PETCO2 (approximately 8–10 mmHg compared to 4–6 mmHg), and such comparisons are made with caution. However, we replicated the approach which has been shown to be acutely sensitive to concussion (Len et al. 2011). Furthermore, CVR–CO2 determined by breath-holding has been shown to be sensitive to sub-concussive head impacts sustained over the course of a season in female footballers (Svaldi et al. 2017). Finally, we observed no alterations in CVR–CO2 following hyperventilation, which did elicit more profound changes in PETCO2 (average change − 12.8 ± 2.3 mmHg) that are consistent with other investigations (Len et al. 2011; Bailey et al. 2013; Owens et al. 2021). Therefore, these data indicate that alterations in CVR–CO2 might not be apparent immediately after three rounds of amateur boxing.
It is important to highlight that our study assessed the influence of three, 3 min rounds of boxing, which is the official length of a competitive British Universities and Colleges Sport amateur boxing bout and consistent with the current format of men’s Olympic boxing. However, a typical sparring session may include more rounds, and such training sessions may occur more than once in a single week. Therefore, understanding the time course in the recovery of dCA metrics would also be a valuable addition to our work. It has been shown that alterations in dCA following subconcussive head impacts may persist for 2 week post-season (Wright et al. 2018a), but no study has yet sought to precisely quantify this time course. This is contextually significant, as there is no standardised recovery time following a competitive bout at amateur level. This concern regarding such habitual exposure to head impacts, and the potentially unachievable length of time required for indices of cerebrovascular function to recover, has been raised elsewhere (Owens et al. 2021). Given that no untoward events or suspected concussions were reported by the club’s medical professional in this study, and that alterations in dCA can occur in the absence of traditional concussion-related symptoms (Wright et al. 2018b), it is important to understand whether head impacts or boxing during a period where dCA is already altered is associated with enhanced sensitivity to cerebrovascular change or heightened risk. To this end, further research should explore the effects of multiple sparring sessions across the week, and how this accumulates across a season, which may be more representative of amateur and professional boxing training.
The present study is the first controlled, experimental study to document metrics of brain blood flow regulation following amateur boxing. The within-measures design and inclusion of a boxing trial without head impacts (PAD) is a considerable methodological strength. However, there are some limitations in our design which should be acknowledged. First, the inclusion criteria outlined that participants only had to have at least 1 year boxing experience. Whilst our cohort are representative of university amateur boxers, this meant that participants were not standardised to skill or experience level. One result of this approach might be the high number of head impacts sustained in three rounds of boxing. It is possible that more skilled boxers may receive fewer blows to the head (Davis et al. 2015). However, it can also be argued that the force of those successful punches might be greater (Doan et al. 2022). These data, therefore, cannot be extrapolated to professional boxing, or other martial arts. Previous interventional studies in this field have attempted to standardise the head impacts—for example, launching a football at a designated speed (Jack et al. 2022; Smirl et al. 2022). In the absence of this control, the inclusion of instrumented mouth guards would be a valuable addition to better describe the nature of head impacts beyond the total number of blows to the head received (i.e., the magnitude and direction of accelerations). This would also help contextualise head impacts in boxing against other sports and provide some insight regarding any possible exposure threshold for changes in dCA. For example, recent data demonstrate that the linear and rotational accelerations of the head experienced during university boxing may be similar to collegiate American football (Doan et al. 2022).
Recent advances in this field have been made, whereby dCA can be separately scrutinised during periods of transient increases and decreases in MAP (Labrecque et al. 2021). This is insightful, as transient hypotension presents a more demanding autoregulatory challenge (Brassard et al. 2017; Labrecque et al. 2021). Unfortunately, it was not possible to consider this directionality in our data, so we cannot comment on whether the alterations in dCA observed are reserved only to periods of transient hypotension or hypertension, or prevail regardless of the direction of MAP change. Providing such insight would be valuable, and should be considered in future studies.
Another shortfall of this study is that it was unable to detect an effect of sex, as only 6 women were recruited. A sex effect on metrics of dCA during SSM is not a consistent finding (Labrecque et al. 2019a; Burma et al. 2020a), but may be particularly relevant in trained women (Labrecque et al. 2019b). Furthermore, women may experience greater brain microtrauma than men following sub-concussive head impacts of a similar magnitude (Rubin et al. 2018), possibly due to differences in neck strength and thus head movement (Caccese et al. 2018). In addition, we report here that the change in phase was not related to years of boxing experience, to broadly consider whether chronic exposure to head impacts might influence the acute response. It has been shown elsewhere that concussion history does not influence the recovery of dCA metrics in the weeks following a concussion in young healthy athletes (Wright et al. 2018b). However, our sample typically had only 3 years of prior boxing experience at amateur level, and far more work is needed to consider this research question. Whether or not a boxer with > 10 years of experience, who may well present with differences in dCA metrics at baseline (Bailey et al. 2013), might demonstrate a different response post boxing remains unknown.
TCD provides valuable temporal resolution when considering the reactivity to changes in arterial pressure and carbon dioxide concentrations. However, the assumption that MCAv is proportional to volumetric flow is contingent on an unchanging vessel diameter (Ainslie and Hoiland 2014). Typically this is accepted when PETCO2 is within ~ 8 mmHg of eucapnia (Coverdale et al. 2014; Verbree et al. 2014). As is typical during SSM (Smirl et al. 2015, 2022; Burma et al. 2020a; Jack et al. 2022), this was achieved during our dCA assessment, but was exceeded during the hyperventilation protocol. However, our hyperventilation challenge replicated a protocol known to be sensitive to change in the days following a concussive event (Len et al. 2011), and we observed similar decreases in PETCO2. Finally, a further shortcoming of our use of TCD is that we could not perform bilateral assessments of cerebral blood velocity due to technological restrictions. Thus, we were unable to quantify the haemodynamic responses beyond the conduit artery insonated (i.e., the MCA). Identifying whether acute head impacts alter brain blood flow regulation in the posterior cerebral circulation remains an interesting avenue for future research.
Conclusion
Using a within-measures, controlled, experimental approach, our study demonstrates that subtle alterations in the cerebral perfusion–pressure relationship can occur in the aftermath of a typical amateur boxing bout. Specifically, dCA phase was lower (i.e., slower) during SSM at 0.05 Hz after 3 rounds of boxing. This change occurred in the absence of any suspected concussive events, and without changes in the cerebrovascular reactivity to carbon dioxide. Further work is now needed to understand the longevity of this perturbation, whether the athlete is more susceptible to further autoregulatory or cerebrovascular changes during this time, and the long term implications of habitual exposure to subconcussive head impacts.
Data availability
The data which support the findings of this study can be made available upon reasonable request.
Abbreviations
- BH:
-
Breath holding
- BHCVR :
-
Percentage change in MCAv per unit change in PETCO2 after each BH attempt
- BHMCAv% :
-
Percentage increase in MCAv above baseline following each BH attempt
- BOX:
-
Boxing trial
- CA:
-
Cerebral autoregulation
- CON:
-
Control trial
- CVR:
-
Cerebrovascular reactivity
- dCA:
-
Dynamic cerebral autoregulation
- HV:
-
Hyperventilation
- HVCVR :
-
Percentage change in MCAv per unit change in PETCO2 after each HV attempt
- HVMCAv% :
-
Percentage increase in MCAv above baseline following each HV attempt
- MAP:
-
Mean arterial pressure
- MCAv:
-
Middle cerebral artery blood velocity
- PAD:
-
Pad-boxing trial
- PETCO2 :
-
Partial pressure of end tidal carbon dioxide
- SSM:
-
Squat-stand manoeuvres
References
Ainslie PN, Hoiland RL (2014) Transcranial Doppler ultrasound: valid, invalid, or both? J Appl Physiol 1985(117):1081–1083
Bailey DM, Jones DW, Sinnott A, Brugniaux JV, New KJ, Hodson D, Marley CJ, Smirl JD, Ogoh S, Ainslie PN (2013) Impaired cerebral haemodynamic function associated with chronic traumatic brain injury in professional boxers. Clin Sci (lond) 124:177–189
Brassard P, Ferland-Dutil H, Smirl JD, Paquette M, Le Blanc O, Malenfant S, Ainslie PN (2017) Evidence for hysteresis in the cerebral pressure-flow relationship in healthy men. Am J Physiol Heart Circ Physiol 312:H701–H704
Burley CV, Lucas RAI, Whittaker AC, Mullinger K, Lucas SJE (2020) The CO2 stimulus duration and steady-state time point used for data extraction alters the cerebrovascular reactivity outcome measure. Exp Physiol 105:893–903
Burma JS, Copeland P, Macaulay A, Khatra O, Smirl JD (2020a) Comparison of diurnal variation, anatomical location, and biological sex within spontaneous and driven dynamic cerebral autoregulation measures. Physiol Rep 8:e14458
Burma JS, Copeland P, Macaulay A, Khatra O, Wright AD, Smirl JD (2020b) Dynamic cerebral autoregulation across the cardiac cycle during 8 hr of recovery from acute exercise. Physiol Rep 8:e14367
Caccese JB, Buckley TA, Tierney RT, Arbogast KB, Rose WC, Glutting JJ, Kaminski TW (2018) Head and neck size and neck strength predict linear and rotational acceleration during purposeful soccer heading. Sports Biomech 17:462–476
Carr J, Caldwell HG, Carter H, Smith K, Tymko MM, Green DJ, Ainslie PN, Hoiland RL (2021) The stability of cerebrovascular CO2 reactivity following attainment of physiological steady-state. Exp Physiol 106:2542–2555
Cohen J (1988) Statistical power analysis for the behavioural sciences, 2nd edn. L Erlbaum Associates, Hillsdale
Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK (2014) Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol 1985(117):1090–1096
Davis P, Benson PR, Pitty JD, Connorton AJ, Waldock R (2015) The activity profile of elite male amateur boxing. Int J Sports Physiol Perform 10:53–57
Doan BK, Heaton KJ, Self BP, Butler Samuels MA, Adam GE (2022) Quantifying head impacts and neurocognitive performance in collegiate boxers. J Sports Sci 40:509–517
England B. England boxing rule book, 2022 edn. https://www.englandboxing.org/wp-content/uploads/2022/01/2022-RULE-BOOK-January-update.pdf
Hamner JW, Tan CO (2014) Relative contributions of sympathetic, cholinergic, and myogenic mechanisms to cerebral autoregulation. Stroke 45:1771–1777
Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA (2010) Sympathetic control of the cerebral vasculature in humans. Stroke 41:102–109
Howe CA, Caldwell HG, Carr J, Nowak-Fluck D, Ainslie PN, Hoiland RL (2020) Cerebrovascular reactivity to carbon dioxide is not influenced by variability in the ventilatory sensitivity to carbon dioxide. Exp Physiol 105:904–915
Huber BR, Alosco ML, Stein TD, McKee AC (2016) Potential long-term consequences of concussive and subconcussive injury. Phys Med Rehabil Clin N Am 27:503–511
Jack J, Woodgates A, Smail O, Brown F, Lynam K, Lester A, Williams G, Bond B (2022) Cerebral blood flow regulation is not acutely altered after a typical number of headers in women footballers. Front Neurol 13:1021536
Koep JL, Barker AR, Banks R, Banger RR, Sansum KM, Weston ME, Bond B (2020) The reliability of a breath-hold protocol to determine cerebrovascular reactivity in adolescents. J Clin Ultrasound 48:544–552
Koep JL, Barker AR, Banks R, Banger RR, Lester A, Sansum KM, Weston ME, Bond B (2021) The acute and postprandial effects of sugar moiety on vascular and metabolic health outcomes in adolescents. Appl Physiol Nutr Metab 46:906–914
Koep JL, Weston ME, Barker AR, Bailey TG, Coombes JS, Lester A, Bond B (2022) The within- and between-day reliability of cerebrovascular reactivity using traditional and novel analytical approaches. Exp Physiol 107:29–41
Labrecque L, Rahimaly K, Imhoff S, Paquette M, Le Blanc O, Malenfant S, Drapeau A, Smirl JD, Bailey DM, Brassard P (2019a) Dynamic cerebral autoregulation is attenuated in young fit women. Physiol Rep 7:e13984
Labrecque L, Smirl JD, Brassard P (2019b) Letter to the Editor: On the need of considering cardiorespiratory fitness when examining the influence of sex on dynamic cerebral autoregulation. Am J Physiol Heart Circ Physiol 316:H1229
Labrecque L, Smirl JD, Brassard P (2021) Utilization of the repeated squat-stand model for studying the directional sensitivity of the cerebral pressure-flow relationship. J Appl Physiol 1985(131):927–936
Lehman EJ, Hein MJ, Baron SL, Gersic CM (2012) Neurodegenerative causes of death among retired National Football League players. Neurology 79:1970–1974
Len TK, Neary JP, Asmundson GJ, Goodman DG, Bjornson B, Bhambhani YN (2011) Cerebrovascular reactivity impairment after sport-induced concussion. Med Sci Sports Exerc 43:2241–2248
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimaki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbaek G, Teri L, Mukadam N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396:413–446
Lota KS, Malliaropoulos N, Blach W, Kamitani T, Ikumi A, Korakakis V, Maffulli N (2022) Rotational head acceleration and traumatic brain injury in combat sports: a systematic review. Br Med Bull 141:33–46
Mackay DF, Russell ER, Stewart K, MacLean JA, Pell JP, Stewart W (2019) Neurodegenerative disease mortality among former professional soccer players. N Engl J Med 381:1801–1808
Marley CJ, Owens TS, Tsukamoto H, Stacey BS, Corkill R, Bailey DM (2021) Impaired cerebral blood flow regulation and cognition in male football players. Scand J Med Sci Sports 31:1908–1913
Narayanan K, Collins JJ, Hamner J, Mukai S, Lipsitz LA (2001) Predicting cerebral blood flow response to orthostatic stress from resting dynamics: effects of healthy aging. Am J Physiol Regul Integr Comp Physiol 281:R716-722
Nowinski CJ, Bureau SC, Buckland ME, Curtis MA, Daneshvar DH, Faull RLM, Grinberg LT, Hill-Yardin EL, Murray HC, Pearce AJ, Suter CM, White AJ, Finkel AM, Cantu RC (2022) Applying the Bradford Hill criteria for causation to repetitive head impacts and chronic traumatic encephalopathy. Front Neurol. https://doi.org/10.3389/fneur.2022.938163
Owens TS, Calverley TA, Stacey BS, Iannatelli A, Venables L, Rose G, Fall L, Tsukamoto H, Berg RMG, Jones GL, Marley CJ, Bailey DM (2021) Contact events in rugby union and the link to reduced cognition: evidence for impaired redox-regulation of cerebrovascular function. Exp Physiol 106:1971–1980
Panerai RB, Brassard P, Burma JS, Castro P, Claassen JA, van Lieshout JJ, Liu J, Lucas SJ, Minhas JS, Mitsis GD, Nogueira RC, Ogoh S, Payne SJ, Rickards CA, Robertson AD, Rodrigues GD, Smirl JD, Simpson DM, Cerebrovascular Research N (2023) Transfer function analysis of dynamic cerebral autoregulation: a CARNet white paper 2022 update. J Cereb Blood Flow Metab 43:3–25
Peng SL, Chu LWL, Su FY (2022) Cerebral hemodynamic response to caffeine: effect of dietary caffeine consumption. NMR Biomed 35:e4727
Pinto J, Bright MG, Bulte DP, Figueiredo P (2020) Cerebrovascular reactivity mapping without gas challenges: a methodological guide. Front Physiol 11:608475
Przybyłowski T, Bangash MF, Reichmuth K, Morgan BJ, Skatrud JB, Dempsey JA (2003) Mechanisms of thecerebrovascular response to apnoea in humans. J Physiol 548(Pt 1):323–332
Rubin TG, Catenaccio E, Fleysher R, Hunter LE, Lubin N, Stewart WF et al (2018) MRI-defined white matter microstructural alteration associated with soccer heading is more extensive in women than men. Radiology 289(2):478–486
Russell ER, Mackay DF, Stewart K, MacLean JA, Pell JP, Stewart W (2021) Association of field position and career length with risk of neurodegenerative disease in male former professional soccer players. JAMA Neurol 78:1057–1063
Smirl JD, Hoffman K, Tzeng YC, Hansen A, Ainslie PN (2015) Methodological comparison of active- and passive-driven oscillations in blood pressure; implications for the assessment of cerebral pressure-flow relationships. J Appl Physiol 1985(119):487–501
Smirl JD, Wright AD, Ainslie PN, Tzeng YC, van Donkelaar P (2018) Differential systolic and diastolic regulation of the cerebral pressure-flow relationship during squat-stand manoeuvres. Acta Neurochir Suppl 126:263–268
Smirl JD, Peacock D, Wright AD, Bouliane KJ, Dierijck J, Burma JS, Kennefick M, Wallace C, van Donkelaar P (2020) An acute bout of soccer heading subtly alters neurovascular coupling metrics. Front Neurol 11:738
Smirl JD, Peacock D, Burma JS, Wright AD, Bouliane KJ, Dierijck J, Kennefick M, Wallace C, van Donkelaar P (2022) An acute bout of controlled subconcussive impacts can alter dynamic cerebral autoregulation indices: a preliminary investigation. Eur J Appl Physiol. https://doi.org/10.1007/s00421-022-04908-4
Svaldi DO, McCuen EC, Joshi C, Robinson ME, Nho Y, Hannemann R et al (2017) Cerebrovascular reactivity changes in asymptomatic female athletes attributable to high school soccer participation. Brain Imaging Behav 11(1):98–112
Tancredi FB, Hoge RD (2013) Comparison of cerebral vascular reactivity measures obtained using breath-holding and CO2 inhalation. J Cereb Blood Flow Metab 33:1066–1074
Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, van Lieshout JJ, van Osch MJ (2014) Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol 1985(117):1084–1089
Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN (2011) Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196:221–237
Wright AD, Smirl JD, Bryk K, Fraser S, Jakovac M, van Donkelaar P (2018a) Cerebral autoregulation is disrupted following a season of contact sports participation. Front Neurol 9:868
Wright AD, Smirl JD, Bryk K, Fraser S, Jakovac M, van Donkelaar P (2018b) Sport-related concussion alters indices of dynamic cerebral autoregulation. Front Neurol 9:196
Zhang J, Yoganandan N, Pintar FA, Gennarelli TA (2006) Role of translational and rotational accelerations on brain strain in lateral head impact. Biomed Sci Instrum 42:501–506
Funding
This work was not supported by external funding.
Author information
Authors and Affiliations
Contributions
The study was conceived by WW and BB. All authors contributed to the study design. Data collection and analysis were performed by WW, QA-A, HL and OS. The first draft of the manuscript was written by WW and BB, and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This work conformed to the guidelines outlined in the 1964 Declaration of Helsinki and received institutional ethical approval from the University of Exeter. All participants provided written, informed consent. The study was not externally funded, and the authors did not receive support from any external organisation. The authors have no conflicts of interest to disclose.
Additional information
Communicated by Ellen Adele Dawson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wallis, W.E.G., Al-Alem, Q., Lorimer, H. et al. The acute influence of amateur boxing on dynamic cerebral autoregulation and cerebrovascular reactivity to carbon dioxide. Eur J Appl Physiol 124, 993–1003 (2024). https://doi.org/10.1007/s00421-023-05324-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05324-y