Abstract
Purpose
Evolving investigative techniques are providing greater understanding about the early neuromuscular responses to resistance training among novice exercisers. The aim of this study was to investigate the time-course of changes in muscle contractile mechanics, architecture, neuromuscular, and strength adaptation during the first 6-weeks of lower-limb resistance training.
Methods
Forty participants: 22 intervention (10 males/12 females; 173.48 ± 5.20 cm; 74.01 ± 13.13 kg) completed 6-week resistance training, and 18 control (10 males/8 females; 175.52 ± 7.64 cm; 70.92 ± 12.73 kg) performed no resistance training and maintained their habitual activity. Radial muscle displacement (Dm) assessed via tensiomyography, knee extension maximal voluntary contraction (MVC), voluntary activation (VA), corticospinal excitability and inhibition via transcranial magnetic stimulation, motor unit (MU) firing rate, and muscle thickness and pennation angle via ultrasonography were assessed before and after 2, 4, and 6-weeks of dynamic lower-limb resistance training or control.
Results
After 2-weeks training, Dm reduced by 19–25% in the intervention group; this was before any changes in neural or morphological measures. After 4-weeks training, MVC increased by 15% along with corticospinal excitability by 16%; however, there was no change in VA, corticospinal inhibition, or MU firing rate. After 6-weeks training there was further MVC increase by 6% along with muscle thickness by 13–16% and pennation angle by 13–14%.
Conclusion
Enhanced contractile properties and corticospinal excitability occurred before any muscle architecture, neural, and strength adaptation. Later increases in muscular strength can be accounted for by architectural adaptation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence suggests that initial training-induced increases in muscle strength are primarily the result of neural adaptation (Komi et al. 1978; Moritani and deVries 1979; Narici et al. 1989; Reeves et al. 2005). Early electromyography (EMG) studies demonstrated an association between increased strength and gross efferent output (Hakkinen and Komi 1983; Aagaard 2003). However, strength training adaptation varies greatly between individuals (Dankel et al. 2020; Roberts et al. 2018) with a cascade of neural, mechanical, and architectural adaptations taking place (Maughan et al. 1983; Higbie et al. 1996; Hubal et al. 2005; Erskine et al. 2010; Blazevich et al. 2008). Now, thanks to technological advancements, specific areas of the neuromuscular pathway can be investigated in isolation, shedding greater light on these initial adaptations. Specifically, individual motor unit (MU) properties (Van Cutsem et al. 1998; Vila-Chã et al. 2010), and parameters of the corticospinal tract and primary motor cortex (M1) (Carroll et al. 2002; Selvanayagam et al. 2011) have been studied. Collectively, these findings suggest that specific locations and responses underpin the early adaptations that typically occur within the first ~ 6 weeks after commencing strength training (Wilson et al. 2019). Observed responses include increased excitability (Goodwill et al. 2012; Weier et al. 2012) and decreased inhibition (Latella et al. 2012) of the corticospinal pathway, as assessed using transcranial magnetic stimulation (TMS) (Kidgell et al. 2017). Such alterations serve to increase motor neuron output and increase force production (i.e., strength) (Kidgell et al. 2017; Siddique et al. 2020). Although, these findings represent one of several areas of potential neural adaptation to resistance training they do not confirm alterations to MU behaviour. Therefore, further investigation, incorporating simultaneous assessment of different regions within the neuromuscular network would provide a more complete understanding of the neural contribution to strength adaptation.
Increased agonist muscle activation resulting from resistance training (Pucci et al. 2006; Jenkins et al. 2017) has been attributed to altered MU behaviour (Van Cutsem et al. 1998); specifically, increased firing rate following periods of resistance training has been detected by intramuscular EMG (Vila-Chã et al. 2010) and by decomposing surface EMG (dEMG) (Del Vecchio et al. 2019). However, conflicting findings have shown unaltered MU firing rate (Rich and Cafarelli 2000; Pucci et al. 2006; Sterczala et al. 2020), reaffirming some of the uncertainty around strength training adaptation. While EMG provides a useful, non-invasive method to assess MU adaptations, it does not allow us to make direct inferences about adaptations within the spinal excitatory/inhibitory networks. Therefore, concurrent measurements of MU discharge properties alongside the associated corticospinal networks of excitation and inhibition could provide clearer insight into the precise nature of the early adaptations to resistance training. To date, similar approaches have been performed in isolation, but as yet, simultaneous measurements across the breadth of the neuromuscular network have not been conducted as part of a robust training intervention paradigm.
We previously demonstrated that tensiomyography- (TMG) derived measurement of muscle belly radial displacement (Dm), which describes the magnitude of muscle deformation in response to percutaneous electrical stimulation, is inversely related to changes in muscle architecture (muscle thickness and fibre pennation angle) (Wilson et al. 2019). When assessing muscle atrophy during bed-rest, Šimunič et al. (2019) demonstrated an increase in Dm prior to any observable change in muscle thickness or pennation angle. This evidence suggests that the time-course of contractile mechanics and architectural changes are not exclusively linked. Although increases in pennation angle and muscle thickness have been shown to contribute to later increases in muscle strength (after > 4-weeks training) (Aagaard et al. 2001; Blazevich et al. 2003), it is not known whether contractile properties would be modified prior to these hypertrophy-orientated architectural adaptations. With early increases in strength primarily attributed to neural adaptations, it may be possible that early alterations in contractile properties are influenced by altered excitation–contraction (E–C) coupling (Calderón et al. 2014). Previously, TMG has been used to infer alterations in E–C coupling following exercise-induced muscle damage (EIMD) (Hunter et al. 2012); where increased contraction time (Tc), the time taken for the muscle belly to reach peak displacement, was associated with secondary EIMD markers. This research demonstrated the capability of TMG to assess early changes in contractile mechanics. To further our understanding of resistance training adaptation it would be beneficial to investigate the time-course of alterations in contractile mechanics in relation to other areas within the neuromuscular pathway. This exploration will provide improved clarity around the underpinning central and peripheral mechanisms responsible for strength adaptation. We anticipate that this research will expand on the growing body of work which challenges some long-held beliefs regarding the neural contributions to resistance training adaptation (Pearcey et al. 2021).
The aim of this study was to track the time-course of changes in muscle contractile properties with relation to muscle architecture and neuromuscular adaptation across a 6-week resistance training programme. By assessing the time-course of changes throughout the neuromuscular pathway we aimed to further elucidate the mechanisms responsible for increasing strength shortly after commencing a new programme of strength training. We hypothesised that over the course of the 6-week resistance training intervention increased strength would be accompanied by reduced Tc and Dm of agonist muscles, indicating improved contractile mechanics and that these contractile adaptations would occur prior to any discernible change to muscle architecture. We additionally hypothesised that increased strength during the initial stages of training would be accompanied by an increase in corticospinal excitability, reduced corticospinal inhibition, and increased MU firing rate.
Methods
Participants
A priori power analysis was conducted (G*power, version 3.1.9.4, Heinrich-Heine University, Dusseldorf, Germany) based on previously published data (increases in 1-RM back squat following 6-weeks resistance training in recreational athletes) (De Souza et al. 2018) and the following parameters: repeated measures analysis of variance (ANOVA), within-between interaction effect size = 0.2, power (1 − β) = 0.8 for the comparison between an experimental group and a control group. The study was not designed to compare within-group response differences between male and female participants. Power analysis showed that 18 participants per group were required to achieve an actual power of 0.81. In total, 43 eligible participants volunteered to take part in the study; 23 participants were randomly assigned to the training group and 20 participants to the control group with groups matched for height and body mass. A higher than indicated number of participants were recruited to account for potential dropouts, based on a similar previous training study (Wilson et al. 2019). Participants were unaccustomed to resistance exercise (i.e., novices) but were otherwise healthy and active. All participants met the UK national guidelines for aerobic physical activity of ≥ 75 min of vigorous activity per week; this was achieved by taking part in team sports 2 × per week, but their training did not involve any form of lower-limb resistance exercise for at least 6 months prior to involvement in the study. Participants were free from musculoskeletal injury or neuromuscular conditions. Prior to study commencement, participants provided informed consent, completed a physical activity readiness questionnaire and the screening questionnaire for TMS (Rossi et al. 2011); no volunteers were excluded for failing to meet the physical activity readiness requirements, one volunteer was excluded prior to testing for failing to pass the TMS screening. The study was approved by the University of Stirling NHS, Invasive or Clinical Research Committee, and all procedures were carried out in accordance with the ethical standards outlined in the latest revision of the Declaration of Helsinki.
Experimental design
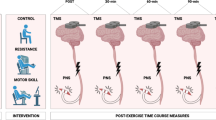
A repeated measures design was used with a separate control group who were instructed to continue with their habitual physical activities and refrain from embarking upon a resistance training programme during the study. The study ran for 9 weeks in total (Fig. 1). Participants attended 2 laboratory sessions in week 1. The first of these laboratory sessions (L1) entailed: ultrasound imaging of muscle architecture, TMG assessment of contractile mechanics, isometric maximal voluntary contraction (MVC), corticospinal excitability/inhibition using TMS, maximal M-wave amplitude (Mmax) and percent of voluntary activation (VA), and dEMG during submaximal isometric contractions. After 48–72 h, the second laboratory session (L2) consisted of a 5-repetition maximum (5-RM) back squat (BS) test. Laboratory sessions carried out in week 1 served as familiarisation sessions, with the same schedule carried out a week later to obtain baseline measurements (Pre-). All measurements were performed by the same skilled investigator. Following Pre- measurements, the intervention group began 6-weeks of lower-limb resistance training (2 × per week) in addition to their habitual physical activity (Table 1). After the 2nd (Wk 2) and 4th (Wk 4) weeks of training, all participants from both groups underwent a repeat of session L1 to obtain time-point measures of any neuromuscular changes. Measurements taken at Wk 2 and Wk 4 were conducted at least 72 h after the previous training session to allow any intramuscular swelling or post-training fatigue in the intervention group to have recovered. Upon completion of the 6-week resistance training programme, all participants underwent repeats of sessions L1 and L2 (~ 4 days after the last training session) to obtain post-intervention measures (Post).
Skeletal muscle architecture and adipose tissue thickness
Ultrasound images of vastus lateralis (VL) and rectus femoris (RF) muscle thickness and pennation angle were obtained from the dominant leg using a 12–4 MHz, 34.5 mm linear transducer (Philips Lumify, NV, USA). Muscle thickness was identified as the distance between the deep and superficial aponeurosis at the ends of each image; pennation angle was identified as the angle between a muscle fascicle and its deep aponeurosis (Fig. 2). Measurement sites of the VL and RF respectively were marked and recorded at 36% of the distance from the superior border of the patella to the anterior superior iliac spine and at 57% of the distance from the superior border of the patella to the anterior superior iliac spine (Wilson et al. 2019). Three longitudinal plane images were taken at each site and exported to Image J (National Institutes of health, Bethesda, MD, USA, version 1.8.0_112) for analysis. Analysis was performed unblinded by the same trained ultrasonographer that performed the scans. Mean values from the three images were used for analysis (Aagaard et al. 2001). Additionally, adipose tissue thickness was measured as the straight-line distance between the top of the facia and bottom of the cutaneous layer (Sterczala et al. 2020). Adipose tissue was measured to account for potential confounding variables which may have impacted EMG recordings throughout the study; adipose tissue is thought to act as a filter on EMG amplitude (Sterczala et al. 2020), and could inadvertently change EMG parameters between time-points.
Tensiomyography
TMG assessment of contractile mechanics was carried out on the VL and RF using the same sites described above for ultrasound imaging, with positioning adjusted to ensure that the thickest part of the muscle belly was being measured (identified by manual palpation of the site). Participants remained in a supine position with the limb rested unfixed on a supportive pad which maintained a knee angle of 60° (0° = full knee extension). A digital TMG sensor (GK 40, Panoptik d.o.o., Ljubljana, Slovenia), with 1 μm sensitivity, was placed perpendicular to the skin surface, with two 5 × 5 cm self-adhesive electrodes (Axelgaard, USA) affixed equidistant on either side of the sensor with a 10 cm inter-electrode distance (centre to centre). The final position of electrodes and sensor were marked and measured with reference to the anterior superior iliac crest and superior border of the patella to allow accurate replication. A single 1 ms wide square wave stimulation (TMG S2, TMG-BMC Ltd., Ljubljana, Slovenia) was applied at an initial amplitude of 20 mA with a constant voltage of 30 V. Amplitude of stimuli was progressively increased by 10 mA, with an inter-stimulus interval of 10–15 s, until a plateau in the displacement–time curve was observed. From the peak twitch-time curve the following parameters were recorded from the TMG software (Version 3.6.16) and taken forward for analysis: maximal radial muscle displacement (Dm) and contraction time (Tc) during the linear portion of the ascending phase of the curve (10–90% of Dm).
Maximal isometric voluntary contraction
Participants were seated in an isokinetic dynamometer with their dominant leg secured to a calibrated load cell (Biodex System 3, Medical Systems, New York, USA). Hip angle was 90° and knee angle was 60° flexion (0° = full knee extension) with the arm of the dynamometer being set such that the axis of rotation was in line with the participant’s lateral femoral epicondyle. Participants remained in this position for all subsequent testing procedures involving isometric contractions (TMS, Mmax, VA, dEMG). Participants were required to perform 3 MVCs of 5 s duration, in response to a randomly timed audio prompt. Participants were instructed to contract as hard and as fast as possible for the duration of the 5 s contraction and received consistent strong verbal encouragement throughout. A minimum of 60 s rest was permitted between MVC attempts. The maximum torque produced within the 3 attempts was used in analysis and for prescription of subsequent testing procedures. We calculated minimal clinically important difference (Lemieux et al. 2007) of strength adaptation from MVC by multiplying the intervention group pooled baseline standard deviation (SD) by 0.2, which corresponds to the smallest effect size. Therefore, our pooled baseline SD was ± 42.6 × 0.2 = 8.5 Nm.
Transcranial magnetic stimulation
Single pulse TMS was used to elicit motor evoked potential (MEP) in RF, assessed using sEMG recordings (detailed below). Single 1 ms stimuli were applied over the contralateral M1 using a magnetic stimulator (Magstim 2002, The Magstim Company Ltd. Whitland, UK) with a 110 mm double cone coil attachment. Optimal coil location for MEP generation was determined by positioning the coil over M1, lateral to the vertex; coil location at which the largest MEP was elicited was identified and marked with semi-permanent ink (Goodall et al. 2009). This position was recorded for replication during subsequent visits. Quadriceps active motor threshold (aMT) was determined by increasing stimulator output in 5% increments, starting from 10%, until a discernible MEP was visible during sustained contraction at an intensity equivalent to 20% of participant MVC achieved during that testing session (Wilson et al. 1995); contraction intensity was maintained by the participant tracing their contraction force to a template visible on a computer monitor directly in front of them. Subsequent stimulations were delivered at 130% of aMT, this protocol has previously demonstrated a high level of reliability in our laboratories (Di Virgilio et al. 2022). A study by Temesi et al. (2014) found no differences between VL, RF, and vastus medialis for aMT or resting MT stimulus–response curves; suggesting that one muscle can be considered representative of all three.
To assess corticospinal excitability, participants contracted at 20% of MVC whilst 20 single pulse stimulations were delivered over M1, separated by 6 s. Corticospinal excitability was determined as the mean MEP peak-to-peak amplitude normalised to the maximal response elicited by motor nerve stimulation (%Mmax, as described below). To assess corticospinal inhibition participants contracted at 20% of MVC for 5 s duration whilst a single stimulation was delivered over M1, as previously reported by our group (Ntikas et al. 2021). This process was repeated 3 times with at least 60 s rest between contractions; mean cortical silent period (cSP) was recorded for analysis. Corticospinal inhibition was quantified as cSP duration, measured from stimulation artefact to the resumption of discernible, uninterrupted EMG activity, identified as an increase of ± 2 SD.
Femoral nerve stimulation
Stimulation of the peripheral femoral nerve was conducted by attaching a self-adhesive surface electrode (cathode) lateral to the femoral artery, high over the femoral triangle, and anode over the gluteus maximus (Newell et al. 2021). Participants applied constant pressure to the cathode using a custom-built spring-loaded algometer to maintain adequate electrode–skin interface; participants were able to maintain consistent pressure on the cathode and reported no difficulties in applying pressure at rest or during contraction. Single stimuli were delivered while participants relaxed (DS7A, Digitimer Ltd., Hertforshire, United Kingdom), and stimulation intensity was incrementally increased until a plateau in twitch amplitude and RF M-wave (Mmax) were observed. The stimulation amplitude was then increased to 120% of the intensity that elicited Mmax and participants received 3 further single stimulations whilst maintaining 20% of MVC to ensure Mmax had been obtained. Next, a further pair of stimulations was delivered to assess VA via twitch interpolation (Merton 1954); first a superimposed stimulation was delivered during MVC (~ 2.5 s after onset of contraction, when maximum force had been attained), followed by a potentiated twitch stimulation 5 s after the superimposed stimulation. Maximum force was taken as the volitional force at the instantaneous delivery of stimulation (Zarkou et al. 2017); since this volitional force may not have always been maximum force level, the correction described by Strojnik and Komi (1998) was applied.
Surface electromyography
Throughout TMS and femoral nerve stimulation protocols, sEMG was recorded from RF of the dominant leg, using a wireless system (Biopac Systems, Inc, Goleta, CA, USA). EMG was sampled at 2 kHz and filtered using 500 Hz low and 1.0 Hz high band filters. All signals were analysed off-line (Acqknowledge, v 3.9.1.6, Biopac Systems, Inc. Goleta, CA, USA). EMG activity was captured using a pair of Ag/AgCl surface electrodes (Ambu Ltd., UK) with inter-electrode distance of 2 cm. Recording site was shaved and abraded prior to electrode placement, as per surface electromyography for the non-invasive assessment of muscles (SENIEM) guidelines; which were also used to determine electrode location on the RF (50% distance on the line between anterior superior iliac spine to the superior border of the patella). A reference electrode was affixed over the patella of the dominant limb. Electrode positions were recorded in reference to anatomical landmarks for accurate replication in all subsequent testing sessions. Resting signals were inspected 80 ms prior to stimulation or voluntary contraction, to ensure the absence of artefact (Brownstein et al. 2017).
Submaximal isometric trapezoid
Following the completion of TMS and peripheral nerve stimulation protocols, surface electrodes were removed, and the skin area cleaned. An electrode array sensor was fixed to VL to capture EMG for decomposition (as described below). Participants performed isometric knee extension following a trapezoidal target trace up to 60% of Pre-MVC. Participants were provided with a visual feedback trace throughout the task, linearly increasing and decreasing torque output at 10% of MVC/s either end of a sustained 10 s hold at 60% of MVC. If the participant failed to adhere to the visual feedback trace, they were allowed a second attempt following 2 min rest. Force equating to 60% of Pre-MVC was used in all subsequent testing sessions.
Decomposition electromyography
A Delsys Trigno Lab wireless system was used to obtain four concurrent sEMG signals for decomposition. A 4-pin surface electrode array (Trigno-Galileo, Delsys, Natick) was affixed to the VL muscle belly, in the same location used for ultrasound imaging and TMG and secured with micropore tape. The reference electrode was affixed over the patella as per manufactures guidelines. VL was selected for measurement due to a typically lower subcutaneous adipose tissue thickness overlying this region compared to RF (reducing the spatial filtering influence of adipose tissue on EMG signals). All four channels of EMG were visually inspected, prior to data recording, to ensure excessive background noise and artefact were not present: baseline noise not exceeding 10 μV according to manufactures guidelines. Signal to noise ratio was calculated within the acquisition software (EMGworks4, Delsys, Natick) according to the following formula: 20log (SRMS/NRMS) [where S = EMG signal and N = baseline noise]. All EMG signals were low pass (fourth-order Butterworth, 24 dB/octave slope, 1750-Hz cut-off) and high pass (second-order Butterworth 12 dB/octave slope, 20-Hz cut-off) filtered prior to sampling at a rate of 20 kHz.
The four separate filtered EMG signals from the sensor array were decomposed into constituent MU action potential trains using Precision Decomposition III (PD III) algorithm (Neuromap, Delsys Inc, Boston, USA). The PD III technique was originally described by Adam and De Luca (2005), and subsequently refined by Nawab et al. (2010). PD III uses artificial intelligence to identify action potential shapes and assign them to individual MUs; with validity and reliability having been previously reported at various contraction intensities up to MVC (Hu et al. 2013). The accuracy of the dEMG process for each trapezoid contraction was assessed by reconstruct and test analysis (Nawab et al. 2010; De Luca and Contessa 2011; De Luca et al. 2015). This analysis assesses the level of firing rate accuracy of each identified MU and the number of errors per second, across the entire contraction (Accuracy = 1 − Nerror/Ntruth; where Nerror is the total number of unmatched events, and Ntruth is total number of true events). Only MUs which displayed > 90% accuracy were included for analysis. Mean firing rate (MFR) curves were smoothed a using 600 ms Hanning window, as recommended by the software manufacturer. From analysis of individual action potential trains, MFR during the constant torque contraction phase (60% of MVC) was calculated. MFR was calculated as the inverse of the averaging inter-spike interval during the distal 3 s of the constant torque phase. This 3 s period had been previously demonstrated in our laboratories to be the most reliable phase for analysis (Balshaw et al. 2017).
5-repetition maximum back squat
In the second lab session (L2), participants completed a 5-RM BS test. 5-RM exercise tests are reliable and valid for recreational athletes of trained and untrained backgrounds (Sascha and Künzell 2014). Following a standardised warm-up, participants completed a structured 5-RM protocol. Participants were permitted up to 3 attempts to obtain their 5-RM. Throughout the session participants reported rating of perceived exertion for each set completed, based on repetitions in reserved (RIR) (Zourdos et al. 2016). These RIR scores were used to guide incremental increases in load lifted for each exercise set. In week 1 (familiarisation), participants were assessed on correct and proper back-squat technique by qualified exercise professionals according to published exercise technical guidelines (Comfort et al. 2018). The 5-RM scores achieved in familiarisation were used to structure the incremental loads for Pre-intervention testing to ensure that 5-RM was achieved within 3 attempts and helped mitigate the learning effect before Pre-intervention measures were taken. Ankle eversion angle and stance width were recorded during baseline testing and replicated in post-intervention testing with the aid of marker tape (Schoenfeld et al. 2016). Participants did not have to complete all 3 attempts if they achieved 5-RM after 1 or 2 attempts. Participants were allowed 2-min rest intervals between sets 1–4, and 4 min between 5-RM attempts. The achieved Pre-intervention 5-RM load was incorporated into the post-intervention testing as the final warm-up set before the first post-intervention 5-RM attempt.
Training intervention
The intervention group completed a 6-week resistance training programme (2 sessions per week, separated by ~ 72 h), consisting of compound lower-limb exercises following a linear periodisation model (Table 1). All training sessions were supervised by qualified instructors to ensure safe technique and correct performance of each exercise. Baseline 5-RM BS score was used to prescribe the loads and increments for each participant’s BS sets, such that participants worked at 80%, 82.5%, and 85% of 5-RM for Session One of Weeks 1–2, 3–4, and 5–6, respectively; training load in Session Two of each week was maintained at 70% of 5-RM for all 6 weeks. A rest period of 3 min was taken between each BS set. All other accessory exercise loads were guided by RIR to ensure sufficient training stimulus (Zourdos et al. 2016), such that training loads were adjusting to achieve a rating of perceived exertion of 6–8 in Weeks 1–3 and of 7–9 in Weeks 4–6, in addition the number of repetitions per set was increased from 8 to 10 to 12 throughout Weeks 1–3 and Weeks 4–6, respectively (Table 1). A rest period of 2 min was taken between each set of each accessory exercise. All participants were provided with drinks containing 20 g whey protein isolate dissolved in water, to aid recovery throughout the 6-week training period (West et al. 2017). Drinks were delivered at the end of each training session to the intervention group, and at the corresponding time points to the control group. To ensure compliance, each drink was provided directly by the investigatory team for both groups.
Statistical analysis
All statistical analysis was carried out using Prism 8 (GraphPad software, CA, USA). Pre-, Wk 2, Wk 4, and Post-intervention data sets were checked for normality (Shapiro–Wilk test). Reliability was calculated from the control group for each dependant variable, using between-session test–retest intraclass correlation coefficient (ICC). For dEMG data, MU firing rate was analysed for each participant on a contraction-by-contraction basis; meaning data were not pooled from multiple contractions, nor multiple participants within a group. Two factor ANOVA with repeated measures was used to determine main effects of training intervention on muscle architecture, contractile mechanics, MVC, VA, corticospinal excitability and inhibition, and dEMG derived MU MFR [2 groups (independent factor) × 4 time-points (repeated measures factor)]. Where significant effect was detected Tukey post hoc analysis was used to identify where significant difference occurred. Cohen’s d effect sizes (ES) were calculated by; d = (Mean1-Mean2)/SDpooled, where SDpooled = √[(SD12 + SD22)/2]. ES are interpreted as: ≤ 0.5 = trivial, 0.5–1.25 = small, 1.25–1.9 = medium, ≥ 2.0 = large, for untrained participants (Rhea 2004). All data are reported as mean and SD, with changes represented as percentage with upper and lower 95% confidence intervals (CI). Alpha was set at P < 0.05. Additionally, since the precise relationship between muscle contractile mechanics and muscle strength is not fully understood, where training-induced changes were observed, Pearson’s correlation coefficients (r) were determined post hoc to describe relationships between strength (MVC and 5-RM) and neuromuscular variables.
Results
Three participants withdrew from the study; 1 from the training group for failing to complete > 90% of training sessions; 2 from the control group, respectively for sustaining an injury unrelated to the study and for failing to pass the TMS screening, leading to a final sample size of 40 participants; 22 in the training group (10 males/12 females; 173.48 ± 5.20 cm; 74.01 ± 13.13 kg; 19.14 ± 1.30 years) and 18 in the control group (10 males/8 females; 175.52 ± 7.64 cm; 70.92 ± 12.73 kg; 20.61 ± 0.94 years).
Strength
Analysis of dynamic strength (5-RM) revealed a significant time effect [F(1,38) = 104.1, P < 0.0001] across both groups, however this was overshadowed by significant Group × Test (4) interaction [F(1,38) = 75.72, P < 0.0001]. The intervention group significantly increased their 5-RM BS strength (P < 0.0001, 95% CI 16.34 to 22.80), with no significant change in the control group (P = 0.53, 95% CI − 2.019 to 5.13). Post-intervention 5-RM BS strength was significantly greater (post hoc analysis) for the intervention group compared to control (P < 0.0001, 95% CI 17.27–43.59) (Table 2). Control group ICC = 0.88. Analysis of isometric strength (MVC) revealed a significant time effect [F(2.54,96.42) = 15.01. P < 0.0001] across both groups, however a significant interaction effect [F(3,114) = 9.297, P < 0.0001] showed the intervention group only significantly increased MVC by Wk 4 (P < 0.001, 95% CI − 42.41 to − 12.06) with further increase between Wk 4 and Post-intervention (P = 0.017, 95% CI − 25.30 to − 2.086). MVC was not significantly different between the intervention and control groups at Wk 4 (P = 0.92, 95% CI − 25 to 45.62) or at Post (P = 0.46, 95% CI − 17.0 to 62.0). Control group ICC = 0.94. Change in MVC from baseline was 27.2 Nm and 40.9 Nm at Wk 4 and Post respectively, thus exceeding the threshold of 8.5 Nm for clinical relevance. This provides confidence that the observed training adaptation substantially exceeds normal MVC variability. Level of VA did not change over time [F(3,114) = 1.11, P = 0.348] nor was there a difference between groups [F(3,114) = 2.60, P = 0.109] (Table 3). Control group ICC = 0.17.
TMG-derived properties
VL Dm showed a significant time effect [F(2.68,101.7) = 6.17, P < 0.01] across both groups, however a significant interaction effect [F(3,114) = 3.811, P = 0.01] revealed the intervention group only significantly reduced VL Dm by Wk 2 (P < 0.001, 95% CI 0.62–2.02). This reduction in VL Dm was maintained at Wk 4 (P = 0.02, 95% CI 0.11–2.06), and at Post (P = 0.01, 95% CI 0.26–2.01) (Fig. 3A). Control group ICC = 0.68.
Time-course measures of contractile properties including: Dm (radial muscle displacement) in the vastus lateralis (VL) (A) and in the rectus femoris (RF) (B); Tc (contraction time) in the VL (C) and RF (D), by group. Individual responses are shown in filled and outline circles, bars display mean ± SD; *significant decrease from pre, P < 0.05; **significant decrease from pre, P < 0.01; ***significant decrease from pre, P < 0.001
Similarly for RF Dm, a significant time effect [F(2.43,92.16) = 3.10, P = 0.04] was observed across both groups, however a significant [F(3,114) = 3.08, P = 0.03] interaction effect showed the intervention group only significantly reduced RF Dm by Wk 2 (P = 0.003, 95% CI 0.47–2.55). This reduction was maintained at Post-intervention (P = 0.004, 95% CI 0.54–3.26), despite not being apparent at Wk 4 (P = 0.17, 95% CI − 0.39 to 3.03) (Fig. 3B). Control group ICC = 0.77.
Neither group significantly changed VL Tc over time [F(2.37,90.01) = 2.17, P = 0.11], no significant interaction effect was found [F(3,114) = 2.48, P = 0.07] (Fig. 3C). Control group ICC = 0.71. Similarly, neither group significantly changed RF Tc over time [F(2.71,102.9) = 1.42, P = 0.24], nor was any significant interaction effect found [F(3,114) = 2.17, P = 0.10] (Fig. 3D). Control group ICC = 0.61. There was no apparent relationship between VL Dm and MVC (r = − 0.102, P = 0.344) or 5-RM (r = − 0.267, P = 0.080), but a significant inverse relationship existed between RF Dm and MVC (r = − 0.245, P = 0.022) but not 5-RM (r = − 0.290, P = 0.057).
Muscle architecture
Muscle architecture data is presented in Table 4. For VL muscle thickness, a significant time effect [F(2.87,109.2) = 27.49, P < 0.0001] was observed across both groups, however a significant interaction effect [F(3,114) = 14.62, P < 0.0001] revealed the intervention group only significantly increased VL muscle thickness by Wk 4 (P = 0.002, 95% CI − 0.24 to -0.05), and then further between Wk 4 and Post (P < 0.0001, 95% CI − 0.29 to − 0.13). Control group ICC = 0.91. Similarly, for RF muscle thickness, a main time effect [F(2.70,102.60) = 15.31, P < 0.0001] was observed across both groups. However a significant interaction effect [F(1,114) = 7.44, P < 0.001] revealed that the intervention group only significantly increased RF muscle thickness Pre-Post (P < 0.0001, 95% CI − 0.41 to − 0.20). Control group ICC = 0.83.
Whilst a time effect [F(2.42,91.82) = 29.04, P < 0.0001] was observed across both groups for VL pennation angle, a significant interaction [F(3,114) = 24.37, P < 0.0001] revealed that the intervention group only increased VL pennation angle Pre-Post (P < 0.0001, 95% CI − 3.46 to − 1.76). Control group ICC = 0.95. Similarly, for RF pennation angle, a time effect [F(1.78,67.44) = 11.14, P < 0.001] was observed across both groups and a significant [F(3,114) = 13.86, P < 0.0001] interaction revealed that the intervention group only significantly increased RF pennation angle Pre-Post (P < 0.001, 95% CI − 3.35 to − 0.99). Control group ICC = 0.92.
TMS-derived properties
A time effect [F(2.51,95.55) = 8,12, P < 0.001] was observed across both groups for corticospinal excitability (MEP amplitude), however, a significant interaction [F(3,114) = 3.93, P = 0.01] revealed that the intervention group only significantly increased corticospinal excitability, by 16% at Wk 4 (P = 0.027, 95% CI − 20.66 to − 0.95); with this increase maintained Post-intervention (21%) (P < 0.001, 95% CI − 22.21 to − 6.53) (Fig. 4A). Representative MEPs at baseline and Wk 4 are presented in Fig. 5. Control group ICC = 0.74. MEP displayed a weak but significant correlation with MVC (r = 0.244, P = 0.022) and a stronger correlation with 5-RM (r = 0.410, p = 0.006).
Time-course measures of corticospinal excitability (A) and corticospinal inhibition (B), assessed by Transcranial magnetic stimulation, by group. Individual responses are shown in filled and outline circles, bars display mean ± SD; *significant increase from pre, P < 0.05; **significant increase from pre, P < 0.01
Unlike corticospinal excitability, corticospinal inhibition (cSP duration) did not significantly [F(2.63,99.93) = 0.49, P = 0.67] change over time and no significant [F(3,114) = 0.69, P = 0.56] interaction effect was found (Fig. 4B). Control group ICC = 0.39.
Motor unit firing rate
Despite the number of MUs identified at each time point being similar between groups (intervention—Pre, 15 ± 6; Wk 2, 16 ± 5; Wk 4, 15 ± 5; Post, 16 ± 4; control – Pre, 16 ± 5; Wk 2, 15 ± 6; Wk 4, 16 ± 5; Post, 16 ± 6), neither group showed a significant change in MU MFR over time [F(2.49,94.78) = 2.70, P = 0.06], and no significant interaction [F(3,114) = 1.89 P = 0.14] was found (Table 5). Control group ICC = 0.41.
Discussion
In the present study we aimed to determine the time-course of neuromuscular adaptations to a resistance training programme among novice participants. Training took place over a 6-week dynamic resistance training period and participants demonstrated increased 5-RM BS supporting the effectiveness of the adopted training programme. A decrease in VL and RF Dm was observed after 2-weeks training and prior to any increase in isometric strength, neural adaptation, or change in muscle architecture. After 4-weeks of training, an increase in maximal quadriceps strength was observed, and accompanied by, increased corticospinal excitability; however, no changes in VA or MU MFR within the quadriceps were seen. Furthermore, an additional increase in maximal quadriceps strength was observed from Wk 4 to Post-intervention, alongside increases in muscle thickness and pennation angle. All effect sizes associated with major findings are presented in Table 6.
Training-induced increases in 5-RM BS strength may be subject to a technique-learning effect; while any perceived strength gain based solely on specific adaptation to a repeatedly trained action should be considered with care, we caution against dismissing initial gains due to potential learning effect, as this effect could still be considered a relevant adaptation (Dankel et al. 2017). Nonetheless, increases in MVC, which we observed at Wk 4 and then again Post-intervention (Table 1), represent an unbiased measure of increased quadriceps strength. In this study, we observed increased quadriceps muscle contractile function and viscoelasticity of the muscle–tendon complex (Evetovich et al. 1997), as shown by decreased VL and RF Dm (Fig. 3A, B). Decreased quadriceps Dm before altered muscle architecture suggests a separate mechanistic change may be responsible for increased muscle tone. A recent study (Šimunič et al. 2019) demonstrated similar decreases in Dm prior to changes in muscle architecture during a recovery intervention following atrophy, also supporting the notion of mechanisms other than architectural adaptation being responsible for altered contractile properties. We previously reported that impaired E-C coupling has been represented by reduced Dm and increased Tc accompanying increased limb girth following EIMD (Hunter et al. 2012). However in the current study, no accompanying change in force output (Table 2) or Tc (Fig. 3C, D) was observed, indicating E-C coupling was not impaired. The present reduction in Dm was ~ 10% less than that shown by Hunter et al. (2012), indicating the less severe form of EIMD that commonly occurs in early stages of resistance training (Damas et al. 2015). A plausible explanation for reduced Dm is the alteration of intra-muscular tissue fluid content (Kasuga 2015) known to occur with EIMD in the early stages of resistance training (Chen et al. 2012). Such a non-invasive marker of contractile function could be useful for practitioners to gain objective insight into the efficacy of training interventions in their early stages. Future work should look to directly measure intra-muscular fluid content changes following resistance training concurrently with changes in contractile properties, to provide precise mechanistic understanding. High reliability of both Dm and Tc has been reported previously, using the same protocol that we have here adopted (Martín-Rodríguez et al. 2017) and the inter-individual spread in Dm recorded in this study is also similar to that reported elsewhere for lower-limb muscles (Llurda-Almuzara et al. 2020). Therefore, while we can be confident that the values at the upper and lower extremes of our range are not the result of measurement error, the significance of relatively high/low Dm within the context of our study is uncertain. This uncertainty arises because TMG measures skeletal muscle contractile mechanics in vivo, meaning that criterion referenced validity is inherently difficult to quantify (Macgregor et al. 2018). That we observed minimal relationships between changes in strength and Dm in both muscles suggests that the variance in Dm is not meaningful. TMG is not without limitations, we have previously raised questions around the external validity of the technique in relation to sports performance (Macgregor et al. 2018). While our current study, along with previous research (Wilson et al. 2019), has revealed associations between TMG-derived parameters and muscle function, it is still evident that the level of force evoked by electrical stimulation using TMG is < 10% of MVC (Ditroilo et al. 2011; Šimunič et al. 2011), which may—at least in part—explain the minimal relationships that we have observed between changes in Dm and strength.
Initial increases in muscular strength after 4-weeks of training were accompanied by neural adaptation in the form of increased MEP amplitude (Fig. 4A), partially confirming our secondary hypothesis. Increased MEP amplitude represents an increase in corticospinal excitability which includes the excitability of M1 and the efficiency of descending volley transmission through the spinal cord and into the muscles (Di Lazzaro et al. 2004). Similar results have previously been shown using dynamic and isometric training interventions, whereby increased corticospinal excitability has accounted for early strength gain (Griffin and Cafarelli 2007; Leung et al. 2013; Mason et al. 2017), although others have observed no change in MEP amplitude (Kidgell and Pearce 2010). Along with the findings of a recent meta-analysis (Siddique et al. 2020) the present study indicates that early-increases in muscular strength can be attributed to improved efficacy of neural transmission along the descending corticospinal tract. Interestingly, whilst corticospinal excitability remained increased (compared to baseline) at Post-intervention assessment, there was no further increase beyond Wk 4 (Fig. 4A). There are limited data regarding adaptations in corticospinal parameters after the initial increases presently observed as, by and large, previous studies have employed 3–5 week training interventions (Kidgell et al. 2017; Siddique et al. 2020). However, present data would suggest corticospinal excitability may not increase further after the initial observed change but rather, remain elevated after 4 weeks of training. We are not the first to report very early elevation in excitation, with little subsequent increase; Mason et al. (2020) reported greater area under the MEP recruitment curve following only one training session, with no further changes. We might speculate that the greater and more distal muscle mass involved in the present study (quadriceps vs. wrist flexors) may explain the delayed plateau in excitability response. Future work investigating the time-course of corticospinal excitability should look to determine the nature of this ‘ceiling effect’ and the influences of new training stimuli, including over a longer training duration to incorporate substantial muscle hypertrophy, and among a variety of muscle groups. The retention of the adaptations we have here observed could additionally be examined by prolonging measurements following cessation of the training stimulus (i.e., a detraining period). Since participants in this study were previously unaccustomed to resistance exercise, our findings are specific to novice exercisers. In this population we have here observed an early plateau in neuromuscular adaptation (i.e., within 6-weeks); whether a similar response could be expected among previously resistance trained individuals—for example, when starting a novel training programme or recommencing training after a period of interruption—is unclear. The principle of muscle memory associated with myonuclear domain size that has been reported in non-human mammalian muscle (Bruusgaard et al. 2012; Gundersen et al. 2018) has not been translated to humans (Psilander et al. 2019) but should not be dismissed as a possible mechanism for augmented adaptation to resistance exercise among non-novices. Therefore, future research should seek to build on our current findings by exploring early responses to resistance exercise in previously trained individuals.
At odds with recent meta-analyses (Kidgell et al. 2017; Siddique et al. 2020), and our own secondary hypothesis, was a lack in change of corticospinal inhibition (Fig. 4B). Recently, Ansdell et al. (2020) also demonstrated no change in cSP duration when assessing short-term training adaptations; however, these authors also saw no change in MEP amplitude unlike the present data. Therefore, it is possible the presently observed results are not due to alterations in M1 but rather somewhere else along the corticospinal tract. However, as TMS MEPs are unable to precisely differentiate between intra-cortical mechanisms (Brownstein et al. 2018) this cannot be confirmed. It should also be acknowledged that whilst shown to be a reliable method of analysis (Damron et al. 2008), cSP measurement does involve practitioner discretion as to when discernible EMG signal re-commences following the silent period; holding a potential for variance. Future work should explore techniques that allow such differentiation to be made within the corticospinal tract, such as stimulation of the cervico-medullary junction to determine efficacy of corticospinal-motor neuronal synapses (Nuzzo et al. 2016). Similarly, despite increases in voluntary strength (5-RM and MVC), we observed no change in VA. Trezise and Blazevich (2019) previously reported increased VA following a longer training programme (10 weeks) of similar frequency to our own, but it is noteworthy that this change showed no relationship with improved isometric strength. While VA may or may not be seen to increase following training, other neuromuscular adaptations are more clearly related to improving strength capability.
In order to gain comprehensive and concurrent insight into early-resistance training adaptations we employed sEMG decomposition to explore MU discharge property adaptations (Rich and Cafarelli 2000; Kamen and Knight 2004). No change in MU MFR, measured at 60% of MVC, was observed at any time point in either group (Table 5). The present MFR data is at odds with a recent study by Del Vecchio et al. (2019) who observed increased MFR in tibialis anterior after 4-weeks isometric training. Disparity between these findings may be due to methodological differences such as the training-specific tests in which MFR was assessed, and the specific equipment (and subsequent algorithms) used to collect MFR data. Another methodological difference was that Del Vecchio et al. (2019) pooled MFR data from multiple contractions, while we did not. Pooling MFR data from multiple contractions has the potential to alter numbers of low-threshold (faster firing) MUs (Van Cutsem et al. 1998) and inadvertently influence MFR. Indeed, MU MFR analysed on a per-contraction basis (as adopted here) suggests MFR is not altered Post-training (Beck et al. 2011; Stock and Thompson 2014; Sterczala et al. 2020). Furthermore, changes in adipose tissue thickness may arbitrarily alter MU properties derived from sEMG due to spatial filtering (Petrofsky 2008); to add a further measure of control to the present study, we measured adipose tissue thickness at the VL and RF electrode sites, with both remaining unchanged over the duration of testing (Table 4). It is possible that resistance training presently altered the recruitment thresholds of MUs, or the degree of MU hypertrophy (Sterczala et al. 2020); but this cannot be confirmed as it was not possible to measure relationships between MU property-recruitment thresholds. Therefore, in line with a previous suggestion (Contessa et al. 2016), future studies should look to these relationships when investigating training induced changes in MU behaviour to obtain greater clarity along the recruitment threshold spectrum.
Later increases in MVC strength (Wk 4 to Post) were accompanied by architectural adaptations in both VL and RF; namely increased pennation angle and muscle thickness (Table 2). Previously, increases in muscle thickness and pennation angle have been demonstrated following similar timeframes of resistance training (Blazevich et al. 2003; DeFreitas et al. 2011; Damas et al. 2015), and have been shown to be contributory to increased capacity for maximal force production (Aagaard et al. 2001; Campbell et al. 2013). It may be beneficial for future studies in this area to also include global measures of body composition to track changes in muscle mass. We observed that VL muscle thickness increased prior to RF (Table 2); this inter-muscle difference possibly resulting from differing stimuli during training (Floyd 2014). Previously, differences in hypertrophic response have been observed between VL and RF (Mangine et al. 2018), with differences in joint articulation involved in the exercises used in the present intervention suggested as explanatory; despite both muscles being controlled by the same innervation point (Page et al. 2019). Despite this inter-muscle difference in adaptation, our findings support the initial hypothesis of muscle architecture enhancements accounting for strength gain in the latter stage of training, as VL muscle thickness also increased from Wk 4 to Post-intervention. Interestingly, VL muscle thickness increased concurrently with corticospinal excitability after 4-weeks training, suggesting that neural and architectural adaptations may not be mutually exclusive. Additionally, corticospinal excitability remained elevated in the presence of further quadriceps architectural adaptation (Wk 4-Post), supporting the notion that a cumulative effect of neural and architectural adaptations account for strength gains. Despite our participants being unaccustomed to resistance exercise, all were healthy and otherwise physically active, as well as being young adults. We know that from the age of ~ 50 years, skeletal muscle mass is progressively lost at a rate of 1–2% per year (Baumgartner et al. 1998; Lauretani et al. 2003) and aged muscle is characterized by smaller muscle fibre diameter, more-varied fibre size, and reduced number of muscle fibres—specifically type II fibres (Frontera et al. 2000; McPhee et al. 2018; Tintignac et al. 2015). In older age, loss of muscle function is greater and more rapid than would be expected from the reduction in muscle mass alone, due in part to motor unit denervation and neuromuscular junction degeneration (McPhee et al. 2018; Mosole et al. 2014). Therefore, we caution against applying our present findings to older adult populations; similarly, further research is required to understand the early responses to novel resistance exercise among sedentary and/or unhealthy population groups.
As previously stated, our study was not designed to compare within-group response differences between male and female participants. Nonetheless, while the present sample (20 males/20 females) may be suitable to infer general conclusions regarding novice resistance exercisers, further research is needed to explore whether inter-sex differences may exist. It is well-understood that male and female responses to resistance exercise differ; men typically maintain ~ 10 kg greater muscle mass than women irrespective of overall body mass (Rossetti et al. 2017), so despite similar relative hypertrophy following resistance training (Abe et al. 2000; Hubal et al. 2005), in absolute terms men can gain up to twice as much muscle mass compared to women (Ivey et al. 2000). Inter-sex differences in TMG-derived properties have been less thoroughly investigated; among healthy individuals, women have been observed to present lower Dm than men in lumbar region (Lohr et al. 2020) and lower-limb musculature (Kusumoto et al. 2023). On the other hand, strong, resistance-trained women displayed greater Dm in lower-limb musculature compared to similarly well-trained men (Herring et al. 2021). Interestingly, Kojić et al. (2021) observed similar decreases in Dm among men and women following a 7-week resistance training intervention, but notably there were no inter-sex differences in Dm before training commenced among those individuals. Taken together, we might speculate that the existing evidence suggests the potential for TMG to distinguish between male and female musculature among healthy, non-resistance trained individuals, but the adaptive response to resistance training appears unlikely to differ between men and women.
Conclusion
We are the first to demonstrate that increased quadriceps muscle contractile function and corticospinal excitability can be observed in the early stages of training prior to increased strength and architectural adaptation. However, precise mechanistic understanding of this change in skeletal muscle contractile mechanics remains to be determined. The integrated assessment approach employed in the present study supports the consensus that early strength is attributed to changes in the neural physiology, however further work is needed to precisely determine the location of adaptation. The cumulative effects of neural and physiological adaptations observed here may provide practitioners with some greater clarity of the time-course aspect of training-induced adaptations. This knowledge may inform the efficacy of training, rehabilitation monitoring and planning considerations.
Data availability
Data will be available upon reasonable request.
Abbreviations
- 5-RM:
-
5-Repetition maximum
- aMT:
-
Active motor threshold
- ANOVA:
-
Analysis of variance
- BS:
-
Back squat
- CI:
-
Confidence interval
- cSP:
-
Cortical silent period
- dEMG:
-
Decomposition electromyography
- Dm:
-
Displacement
- E-C coupling:
-
Excitation-contraction coupling
- EIMD:
-
Exercise-induced muscle damage
- EMG:
-
Electromyography
- ES:
-
Effect size
- ICC:
-
Intraclass correlation coefficient
- M1:
-
Motor cortex
- MEP:
-
Motor evoked potential
- MFR:
-
Mean firing rate
- Mmax :
-
Maximal M-wave amplitude
- MU:
-
Motor unit
- MVC:
-
Maximal voluntary contraction
- PD III:
-
Precision Decomposition III
- RF:
-
Rectus femoris
- RIR:
-
Repetitions in reserve
- SD:
-
Standard deviation
- Tc:
-
Contraction time
- TMG:
-
Tensiomyography
- TMS:
-
Transcranial magnetic stimulation
- VA:
-
Voluntary activation
- VL:
-
Vastus lateralis
References
Aagaard P (2003) Training-induced changes in neural function. Exerc Sport Sci Rev 31:61–67
Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers A-M, Wagner A, Magnusson SP, Halkjær-Kristensen J, Simonsen EB (2001) A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol 534:613–623
Abe T, DeHoyos DV, Pollock ML, Garzarella L (2000) Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol 81:174–180
Adam A, De Luca CJ (2005) Firing rates of motor units in human vastus lateralis muscle during fatiguing isometric contractions. J Appl Physiol 99:268–280
Ansdell P, Brownstein CG, Škarabot J, Angius L, Kidgell D, Frazer A, Hicks KM, Durbaba R, Howatson G, Goodall S, Thomas K (2020) Task-specific strength increases after lower-limb compound resistance training occurred in the absence of corticospinal changes in vastus lateralis. Exp Physiol 105:1132–1150
Balshaw TG, Pahar M, Chesham R, Macgregor LJ, Hunter AM (2017) Reduced firing rates of high threshold motor units in response to eccentric overload. Physiol Rep 5:e13111
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147(8):755–763
Beck, TW, DeFreitas, JM, Stock, MS (2011) The effects of a resistance training program on average motor unit firing rates. Clinical Kinesiology (Online Edition)
Blazevich AJ, Gill ND, Bronks R, Newton RU (2003) Training-specific muscle architecture adaptation after 5-wk training in athletes. Med Sci Sports Exerc 35:2013–2022
Blazevich AJ, Horne S, Cannavan D, Coleman DR, Aagaard P (2008) Effect of contraction mode of slow-speed resistance training on the maximum rate of force development in the human quadriceps. Muscle Nerve 38:1133–1046
Brownstein CG, Dent JP, Parker P, Hicks KM, Howatson G, Goodall S, Thomas K (2017) Etiology and recovery of neuromuscular fatigue following competitive soccer match-play. Front Physiol 8:831
Brownstein CG, Ansdell P, Škarabot J, Frazer A, Kidgell D, Howatson G, Goodall S, Thomas K (2018) Motor cortical and corticospinal function differ during an isometric squat compared with isometric knee extension. Exp Physiol 103:1251–1263
Bruusgaard JC, Egner IM, Larsen TK, Dupre-Aucouturier S, Desplanches D, Gundersen K (2012) No change in myonuclear number during muscle unloading and reloading. J Appl Physiol 113(2):290–296
Calderón JC, Bolaños P, Caputo C (2014) The excitation-contraction coupling mechanism in skeletal muscle. Biophys Rev 6:133–160
Campbell EL, Seynnes OR, Bottinelli R, Mcphee JS, Atherton PJ, Jones DA, Butler-Browne G, Narici MV (2013) Skeletal muscle adaptations to physical inactivity and subsequent retraining in young men. Biogerontology 14:247–259
Carroll TJ, Riek S, Carson RG (2002) The sites of neural adaptation induced by resistance training in humans. J Physiol 544:641–652
Chen HL, Nosaka K, Pearce AJ, Chen TC (2012) Two maximal isometric contractions attenuate the magnitude of eccentric exercise-induced muscle damage. Appl Physiol Nutr Metab 37:680–689
Comfort P, Mcmahon JJ, Suchomel TJ (2018) Optimizing squat technique-revisited. Strength Cond J 29:10–13
Contessa P, De Luca CJ, Kline JC (2016) The compensatory interaction between motor unit firing behavior and muscle force during fatigue. J Neurophysiol 116:1579–1585
Damas F, Phillips SM, Lixandrão ME, Vechin FC, Libardi CA, Roschel H, Tricoli V, Ugrinowitsch C (2015) Early resistance training-induced increases in muscle cross-sectional area are concomitant with edema-induced muscle swelling. Eur J Appl Physiol 116:49–56
Damron LA, Dearth DJ, Hoffman RL, Clark BC (2008) Quantification of the corticospinal silent period evoked via transcranial magnetic stimulation. J Neurosci Methods 173:121–128
Dankel SJ, Counts BR, Barnett BE, Buckner SL, Abe T, Loenneke JP (2017) Muscle adaptations following 21 consecutive days of strength test familiarization compared with traditional training. Muscle Nerve 56:307–314
Dankel SJ, Bell ZW, Spitz RW, Wong V, Viana RB, Chatakondi RN, Buckner SL, Jessee MB, Mattocks KT, Mouser JG, Abe T, Loenneke JP (2020) Assessing differential responders and mean changes in muscle size, strength, and the crossover effect to 2 distinct resistance training protocols. Appl Physiol Nutr Metab 45(5):463–470
De Luca CJ, Contessa P (2011) Hierarchical control of motor units in voluntary contractions. J Neurophysiol 107:178–195
De Luca CJ, Nawab SH, Kline JC (2015) Clarification of methods used to validate surface EMG decomposition algorithms as described by Farina et al. (2014). J Appl Physiol 118:1084–1084
De Souza EO, Tricoli V, Rauch J, Alvarez MR, Laurentino G, Aihara AY, Cardoso FN, Roschel H, Ugrinowitsch C (2018) Different patterns in muscular strength and hypertrophy adaptations in untrained individuals undergoing nonperiodized and periodized strength regimens. J Strength Condit Res 32:1238–1244
Defreitas JM, Beck TW, Stock MS, Dillon MA, Kasishke PR (2011) An examination of the time course of training-induced skeletal muscle hypertrophy. Eur J Appl Physiol 111:2785–2790
Del Vecchio A, Casolo A, Negro F, Scorcelletti M, Bazzucchi I, Enoka R, Felici F, Farina D (2019) The increase in muscle force after 4 weeks of strength training is mediated by adaptations in motor unit recruitment and rate coding. J Physiol 597:1873–1887
Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC (2004) The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 115:255–266
Di Virgilio TG, Ietswaart M, Selvamoorthy R, Hunter AM (2022) The reliability of transcranial magnetic stimulation-derived corticomotor inhibition as a brain health evaluation tool in soccer players. Sports Med Open 8:1–9
Ditroilo M, Hunter AM, Haslam S, De Vito G (2011) The effectiveness of two novel techniques in establishing the mechanical and contractile responses of biceps femoris. Physiol Meas 32(8):1315–1326
Evetovich TK, Housh TJ, Stout JR, Johnson GO, Smith DB, Ebersole KT (1997) Mechanomyographic responses to concentric isokinetic muscle contractions. Eur J Appl Physiol 75:166–169
Erskine RM, Jones DA, Williams AG, Stewart CE, Degens H (2010) Inter-individual variability in the adaptation of human muscle specific tension to progressive resistance training. Eur J Appl Physiol 110:1117–1125
Floyd RT, Thompson CW (2009) Manual of structural kinesiology, vol 16. McGraw-Hill, New York, NY
Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R (2000) Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol 88(4):1321–1326
Goodall S, Romer LM, Ross EZ (2009) Voluntary activation of human knee extensors measured using transcranial magnetic stimulation. Exp Physiol 94:995–1004
Goodwill AM, Pearce AJ, Kidgell DJ (2012) Corticomotor plasticity following unilateral strength training. Muscle Nerve 46:384–393
Griffin L, Cafarelli E (2007) Transcranial magnetic stimulation during resistance training of the tibialis anterior muscle. J Electromyogr Kinesiol 17:446–452
Gundersen K, Bruusgaard JC, Egner IM, Eftestøl E, Bengtsen M (2018) Muscle memory: virtues of your youth? J Physiol 596(18):4289
Hakkinen K, Komi PV (1983) Electromyographic changes during strength training and detraining. Med Sci Sports Exerc 15:455–460
Herring CH, Goldstein ER, Fukuda DH (2021) Use of tensiomyography in evaluating sex-based differences in resistance-trained individuals after plyometric and isometric midthigh pull postactivation potentiation protocols. J Strength Condit Res 35(6):1527–1534
Higbie EJ, Cureton KJ, Warren GL III, Prior BM (1996) Effects of concentric and eccentric training on muscle strength, cross-sectional area, and neural activation. J Appl Physiol 81:2173–2181
Hu X, Rymer WZ, Suresh NL (2013) Assessment of validity of a high-yield surface electromyogram decomposition. J Neuroeng Rehabil 10:99
Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Clarkson PM (2005) Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc 37:964–972
Hunter AM, Galloway SDR, Smith IJ, Tallent J, Ditroilo M, Fairweather MM, Howatson G (2012) Assessment of eccentric exercise-induced muscle damage of the elbow flexors by tensiomyography. J Electromyogr Kinesiol 22:334–341
Ivey FM, Roth SM, Ferrell RE, Tracy BL, Lemmer JT, Hurlbut DE, Martel GF, Siegel EL, Fozard JL, Jeffrey Metter E, Fleg JL, Hurley BF (2000) Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol Ser A Biol Sci Med Sci 55:M641–M648
Jenkins NDM, Miramonti AA, Hill EC, Smith CM, Cochrane-Snyman KC, Housh TJ, Cramer JT (2017) Greater neural adaptations following high- vs. low-load resistance training. Front Physiol 8:331
Kamen G, Knight CA (2004) Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol Ser A 59:1334–1338
Kasuga N (2015) Adaptative changes and contractile properties of skeletal muscle: Significance and problems of tension measurement. J Phys Fitness Sports Med 4:343–346
Kidgell DJ, Pearce AJ (2010) Corticospinal properties following short-term strength training of an intrinsic hand muscle. Hum Mov Sci 29:631–641
Kidgell DJ, Bonanno DLR, Frazer AK, Howatson G, Pearce AJ (2017) Corticospinal responses following strength training: a systematic review and meta-analysis. Eur J Neurosci 46:2648–2661
Kojić F, Mandić D, Ilić V (2021) Resistance training induces similar adaptations of upper and lower-body muscles between sexes. Sci Rep 11(1):23449
Komi PV, Viitasalo JT, Rauramaa R, Vihko V (1978) Effect of isometric strength training on mechanical, electrical, and metabolic aspects of muscle function. Eur J Appl Physiol 40:45–55
Kusumoto Y, Goto H, Chiba K, Oonishi S, Tsuchiya J (2023) Characteristics of muscle contraction of the rectus femoris using tensiomyography by sex in healthy college students: a cross-sectional study. PeerJ 11:e14732
Latella C, Kidgell DJ, Pearce AJ (2012) Reduction in corticospinal inhibition in the trained and untrained limb following unilateral leg strength training. Eur J Appl Physiol 112:3097–3107
Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L (2003) Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 95(5):1851–1860
Lemieux J, Beaton DE, Hogg-Johnson S, Bordeleau LJ, Goodwin PJ (2007) Three methods for minimally important difference: no relationship was found with the net proportion of patients improving. J Clin Epidemiol 60(5):448–455
Leung M, Spittle M, Kidgell DJ (2013) Corticospinal excitability following short-term motor imagery training of a strength task. J Imagery Res Sport Phys Activ 8:35–44
Llurda-Almuzara L, Pérez-Bellmunt A, López-de-Celis C, Aiguadé R, Seijas R, Casasayas-Cos O, Labata-Lezaun N, Alvarez P (2020) Normative data and correlation between dynamic knee valgus and neuromuscular response among healthy active males: a cross-sectional study. Sci Rep 10(1):17206
Lohr C, Schmidt T, Braumann KM, Reer R, Medina-Porqueres I (2020) Sex-based differences in tensiomyography as assessed in the lower erector spinae of healthy participants: an observational study. Sports Health 12(4):341–346
Macgregor LJ, Hunter AM, Orizio C, Fairweather MM, Ditroilo M (2018) Assessment of skeletal muscle contractile properties by radial displacement: the case for tensiomyography. Sports Med 48:1607–1620
Mangine GT, Redd MJ, Gonzalez AM, Townsend JR, Wells AJ, Jajtner AR, Beyer KS, Boone CH, La Monica MB, Stout JR, Fukuda DH, Ratamess NA, Hoffman JR (2018) Resistance training does not induce uniform adaptations to quadriceps. PLoS ONE 13:e0198304
Martín-Rodríguez S, Loturco I, Hunter AM, Rodríguez-Ruiz D, Munguia-Izquierdo D (2017) Reliability and measurement error of tensiomyography to assess mechanical muscle function: a systematic review. J Strength Condit Res 31(12):3524–3536
Mason J, Frazer A, Horvath DM, Pearce AJ, Avela J, Howatson G, Kidgell D (2017) Adaptations in corticospinal excitability and inhibition are not spatially confined to the agonist muscle following strength training. Eur J Appl Physiol 117:1359–1371
Mason J, Frazer AK, Avela J, Pearce AJ, Howatson G, Kidgell DJ (2020) Tracking the corticospinal responses to strength training. Eur J Appl Physiol 120(4):783–798
Maughan RJ, Watson JS, Weir J (1983) Strength and cross-sectional area of human skeletal muscle. J Physiol 338:37–49
McPhee JS, Cameron J, Maden-Wilkinson T, Piasecki M, Yap MH, Jones DA, Degens H (2018) The contributions of fiber atrophy, fiber loss, in situ specific force, and voluntary activation to weakness in sarcopenia. J Gerontol Ser A 73(10):1287–1294
Merton PA (1954) Voluntary strength and fatigue. J Physiol 123:553–564
Moritani T, Devries HA (1979) Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med Rehabil 58:115–130
Mosole S, Carraro U, Kern H, Loefler S, Fruhmann H, Vogelauer M, Burggraf S, Mayr W, Krenn M, Paternostro-Sluga T, Hamar D, Cvecka J, Sedliak M, Tirpakova V, Sarabon N, Musaró A, Sandri M, Protasi F, Nori A, Pond A, Zampieri S (2014) Long-term high-level exercise promotes muscle reinnervation with age. J Neuropathol Exp Neurol 73(4):284–294
Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P (1989) Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol Occup Physiol 59:301–319
Nawab SH, Chang S-S, De Luca CJ (2010) High-yield decomposition of surface EMG signals. Clin Neurophysiol 121:1602–1615
Newell ML, Macgregor LJ, Galloway SDR, Hunter AM (2021) Prolonged cycling exercise alters neural control strategy, irrespective of carbohydrate dose ingested. Transl Sports Med 4:88–99
Ntikas M, Hunter AM, Gallagher IJ, Di Virgilio TG (2021) Longer neurophysiological vs. clinical recovery following sport concussion. Front Sports Active Liv 3:737712
Nuzzo JL, Barry BK, Gandevia SC, Taylor JL (2016) Acute Strength Training Increases Responses to Stimulation of Corticospinal Axons. Med Sci Sports Exerc 48:139–150
Page BJ, Mrowczynski OD, Payne RA, Tilden SE, Lopez H, Rizk E, Harbaugh K (2019) The relative location of the major femoral nerve motor branches in the thigh. Cureus 11:e3882–e3882
Pearcey GEP, Alizedah S, Power KE, Button DC (2021) Chronic resistance training: is it time to rethink the time course of neural contributions to strength gain? Eur J Appl Physiol 121:2413–2422
Petrofsky J (2008) The effect of the subcutaneous fat on the transfer of current through skin and into muscle. Med Eng Phys 30:1168–1176
Psilander N, Eftestøl E, Cumming KT, Juvkam I, Ekblom MM, Sunding K, Wernbom M, Holmberg H-C, Ekblom B, Bruusgaard JC, Raastad T, Gundersen K (2019) Effects of training, detraining, and retraining on strength, hypertrophy, and myonuclear number in human skeletal muscle. J Appl Physiol 126:1636–1645
Pucci AR, Griffin L, Cafarelli E (2006) Maximal motor unit firing rates during isometric resistance training in men. Exp Physiol 91:171–178
Reeves ND, Maganaris CN, Narici MV (2005) Plasticity of dynamic muscle performance with strength training in elderly humans. Muscle Nerve 31:355–364
Rhea MR (2004) Determining the magnitude of treatment effects in strength training research through the use of the effect size. J Strength Condit Res 18:918–920
Rich C, Cafarelli E (2000) Submaximal motor unit firing rates after 8 wk of isometric resistance training. Med Sci Sports Exerc 32:190–196
Roberts MD, Haun CT, Mobley CB, Mumford PW, Romero MA, Roberson PA, Vann CG, McCarthy JJ (2018) Physiological differences between low versus high skeletal muscle hypertrophic responders to resistance exercise training: current perspectives and future research directions. Front Physiol 9:834
Rossetti ML, Steiner JL, Gordon BS (2017) Androgen-mediated regulation of skeletal muscle protein balance. Mol Cell Endocrinol 447:35–44
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2011) Screening questionnaire before TMS: an update. Clin Neurophysiol 122:1686
Sascha G, Künzell S (2014) Reliability of a 5-repetition maximum strength test in recreational athletes. Dtsch Z Sportmed 65:314–317
Schoenfeld BJ, Ogborn D, Krieger JW (2016) Effects of resistance training frequency on measures of muscle hypertrophy: a systematic review and meta-analysis. Sports Med 46:1689–1697
Selvanayagam VS, Riek S, Carroll TJ (2011) Early neural responses to strength training. J Appl Physiol 111:367–375
Siddique U, Rahman S, Frazer AK, Pearce AJ, Howatson G, Kidgell DJ (2020) Determining the sites of neural adaptations to resistance training: a systematic review and meta-analysis. Sports Med 50:1107–1128
Šimunič B, Degens H, Rittweger J, Narici M, Mekjavic IB, Pišot R (2011) Noninvasive estimation of myosin heavy chain composition in human skeletal muscle. Med Sci Sports Exerc 43(9):1619–1625
Šimunič B, Koren K, Rittweger J, Lazzer S, Reggiani C, Rejc E, Pišot R, Narici M, Degens H (2019) Tensiomyography detects early hallmarks of bed-rest-induced atrophy before changes in muscle architecture. J Appl Physiol 126:815–822
Sterczala AJ, Miller JD, Dimmick HL, Wray ME, Trevino MA, Herda TJ (2020) Eight weeks of resistance training increases strength, muscle cross-sectional area and motor unit size, but does not alter firing rates in the vastus lateralis. Eur J Appl Physiol 120:281–294
Stock MS, Thompson BJ (2014) Effects of barbell deadlift training on submaximal motor unit firing rates for the vastus lateralis and rectus femoris. PLoS ONE 9:e115567
Strojnik V, Komi PV (1998) Neuromuscular fatigue after maximal stretch-shortening cycle exercise. J Appl Physiol 84(1):344–350
Temesi J, Gruet M, Rupp T, Verges S, Millet GY (2014) Resting and active motor thresholds versus stimulus–response curves to determine transcranial magnetic stimulation intensity in quadriceps femoris. J Neuroeng Rehabil 11:40
Tintignac LA, Brenner HR, Rüegg MA (2015) Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev 95(3):809–852
Trezise J, Blazevich AJ (2019) Anatomical and neuromuscular determinants of strength change in previously untrained men following heavy strength training. Front Physiol 10:1001
Van Cutsem M, Duchateau J, Hainaut K (1998) Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol 513:295–305
Vila-Chã C, Falla D, Farina D (2010) Motor unit behavior during submaximal contractions following six weeks of either endurance or strength training. J Appl Physiol 109:1455–1466
Weier AT, Pearce AJ, Kidgell DJ (2012) Strength training reduces intracortical inhibition. Acta Physiol 206:109–119
West DWD, Abou Sawan S, Mazzulla M, Williamson E, Moore DR (2017) Whey protein supplementation enhances whole body protein metabolism and performance recovery after resistance exercise: a double-blind crossover study. Nutrients 9:735
Wilson SA, Thickbroom GWF, Mastaglia FL, Mastaglia FL (1995) Comparison of the magnetically mapped corticomotor representation of a muscle at rest and during low-level voluntary contraction. Electroencephalogr Clin Neurophysiol 97:246–250
Wilson MT, Ryan AMF, Vallance SR, Dias-Dougan A, Dugdale JH, Hunter AM, Hamilton DL, Macgregor LJ (2019) Tensiomyography derived parameters reflect skeletal muscle architectural adaptations following 6-weeks of lower body resistance training. Front Physiol 10:1493
Zarkou A, Stackhouse S, Binder-Macleod SA, Lee SC (2017) Comparison of techniques to determine human skeletal muscle voluntary activation. J Electromyogr Kinesiol 36:8–15
Zourdos MC, Klemp A, Dolan C, Quiles JM, Schau KA, Jo E, Helms E, Esgro B, Duncan S, Garcia Merino S, Blanco R (2016) Novel resistance training-specific rating of perceived exertion scale measuring repetitions in reserve. J Strength Condit Res 30:267–275
Acknowledgements
The authors would like to acknowledge sport scotland institute of sport for their support throughout the study. The authors would also like to acknowledge Arla Foods for providing recovery drinks. Finally, we thank the participants for volunteering their time to take part in the study.
Funding
This work was funded by sportscotland to Angus Murray Hunter with grant number TMG_42.
Author information
Authors and Affiliations
Contributions
AH and LM conceived and designed the research. MW conducted the experiment and performed analysis and write up with input and advice from AH, LM, LH and MF. Ultrasound methodology and analysis was provided by SK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Communicated by Philip D. Chilibeck.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wilson, M.T., Hunter, A.M., Fairweather, M. et al. Enhanced skeletal muscle contractile function and corticospinal excitability precede strength and architectural adaptations during lower-limb resistance training. Eur J Appl Physiol 123, 1911–1928 (2023). https://doi.org/10.1007/s00421-023-05201-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05201-8