Abstract
Purpose
τ of the primary phase of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics during square-wave, moderate-intensity exercise mirrors that of PCr splitting (τPCr). Pre-exercise [PCr] and the absolute variations of PCr (∆[PCr]) occurring during transient have been suggested to control τPCr and, in turn, to modulate \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics. In addition, \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics may be slower when exercise initiates from a raised metabolic level, i.e., from a less-favorable energetic state. We verified the hypothesis that: (i) pre-exercise [PCr], (ii) pre-exercise metabolic rate, or (iii) ∆[PCr] may affect the kinetics of muscular oxidative metabolism and, therefore, τ.
Methods
To this aim, seven active males (23.0 yy ± 2.3; 1.76 m ± 0.06, \(\dot{V}{\text{O}}_{2\max }\): 3.32 L min−1 ± 0.67) performed three repetitions of series consisting of six 6-min step exercise transitions of identical workload interspersed with different times of recovery: 30, 60, 90, 120, 300 s.
Results
Mono-exponential fitting was applied to breath-by-breath \(\dot{V}{\text{O}}_{{2{\text{A}}}}\), so that τ was determined. τ decays as a first-order exponential function of the time of recovery (τ = 109.5 × e(−t/14.0) + 18.9 r2 = 0.32) and linearly decreased as a function of the estimated pre-exercise [PCr] (τ = − 1.07 [PCr] + 44.9, r2 = 0.513, P < 0.01); it was unaffected by the estimated ∆[PCr].
Conclusions
Our results in vivo do not confirm the positive linear relationship between τ and pre-exercise [PCr] and ∆[PCr]. Instead, \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics seems to be influenced by the pre-exercise metabolic rate and the altered intramuscular energetic state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alveolar oxygen uptake (\(\dot{V}{\text{O}}_{{2{\text{A}}}}\)) at the onset of constant-load, moderate-intensity exercise attains the steady-state value (\(\dot{V}{\text{O}}_{{2{\text{ss}}}}\)) following kinetics commonly described as the sum of two mono-exponential responses (Whipp and Ward 1990). The first component (Phase 1, or the cardio-dynamic phase) is related to the rapid increment of pulmonary blood flow (Lador et al. 2006; Loeppke et al. 1981; Yoshida et al. 1993). The second (Phase 2, or primary phase): (i) mirrors the O2 uptake response of the muscles (\(\dot{V}{\text{O}}_{{2{\text{m}}}}\)) (Grassi et al. 1996, 1998), (ii) appears after a time delay of about 15 s, and (iii) is characterized by a time constant (τ2) of around 35–45 s in moderately fit healthy subjects (Poole and Jones 2005).
The slowness of \(\dot{V}{\text{O}}_{{2{\text{m}}}}\) kinetics at the onset of exercise implies that the fraction of ATP resynthesized from phosphocreatine (PCr) breakdown before achieving \(\dot{V}{\text{O}}_{{2{\text{ss}}}}\) progressively decreases. The amount of energy derived from the splitting of PCr, the biochemical counterpart of the obligatory O2 deficit (DefO2) (Ferretti et al. 2022; di Prampero 1981; Piiper et al. 1968), fills the gap between the energy requirement of muscle contraction and the energy provided by the aerobic pathway, and is linearly related to \(\dot{V}{\text{O}}_{{2{\text{ss}}}}\). This phenomenon implies that \(\dot{V}{\text{O}}_{{2{\text{m}}}}\) and phosphocreatine concentration ([PCr]) attain the corresponding steady states in a mirror fashion following mono-exponential functions characterized by similar τ (McCreary et al. 1996; Rossiter et al. 1999; Whipp et al. 1999).
The factors regulating the kinetics of \(\dot{V}{\text{O}}_{{2{\text{m}}}}\) and of PCr splitting have been investigated in detail by applying several experimental approaches, and various models of the regulation of the mitochondrial respiration in skeletal muscles have been explained (Meyer 1988; Grassi 2003; Haseler et al. 2004; Kindig et al. 2005). Korzeniewski and Zoladz, for instance, proposed a dynamical model of the respiratory control in muscle fibers based on the quantitative integration of kinetic equations, features of putative enzymes, and biochemical pathways-blocks (Korzeniewski and Zoladz 2001). According to this model, the τ of muscular PCr splitting (τPCr) (and therefore of muscular and alveolar \(\dot{V}{\text{O}}_{2}\) kinetics) is related to the decrease of PCr during the rest-to-work transition (∆PCr) in such a way that τPCr increases linearly as a function of ∆PCr (Korzeniewski and Zoladz 2004). The authors, in agreement with their hypothesis, state that “after the termination of an exercise, if [PCr] did not return to its resting level and a new exercise is started, ∆PCr for this principal exercise will, of course, be smaller than for the previous exercise and therefore t1/2 also will be smaller.” (Fig. 5 and page 708, Korzeniewski and Zoladz 2004). In compliance with the aforementioned theoretical model, experimental data showed a correlation between τPCr at the onset of square-wave exercise and ∆PCr (Greiner et al. 2007). However, more recent findings, based on 31P-NMR spectroscopy measures of PCr breakdown at the onset of moderate-intensity, square-wave exercise, did not confirm a direct relationship between ∆PCr and the speed of PCr splitting (Francescato et al. 2008). Instead, these data suggested that [PCr] is the primary controller of oxidative phosphorylation in the skeletal muscle (Walsh et al. 2001) and acts as a sort of energy buffer with a capacitance that is proportional to the PCr content (Meyer 1989). Consequently, τPCr is slower (longer in time), the more prominent the [PCr] prevailing before exercise onset.
At the end of the exercise, the energy requirement and the ATP splitting rate diminish immediately. Nevertheless, \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) gradually decreases following complex kinetics, usually described as the sum of two mono-exponential decays (Cautero et al. 2005). The rapid component of this kinetics mirrors the rate of PCr resynthesis, and the consumed O2 is utilized to repay the DefO2 contracted at the onset of the exercise (Piiper and Spiller 1970). The τ of this decay (35–40 s) implies that DefO2 is completely repaid in about 175–200 s, a time likely sufficient for the full recovery of [PCr]. This recovery time also suggests that if the exercise is resumed before the obligatory DefO2 has been fully repaid and PCr stores have not been wholly replenished, we start exercising from a lower [PCr]. Suppose the metabolic requirement, i.e., the \(\dot{V}{\text{O}}_{{2{\text{ss}}}}\) elicited by the imposed workload, is unchanged. In that case, we also necessarily imply that the ∆PCr induced by the step transition is lower than that observed when starting from rest. Therefore, the imposition of a square-wave transition at identical workloads, but after different recovery times, might be a simple and effective method to manipulate in a predetermined fashion the pre-transition [PCr] and the ∆PCr of square-wave exercise and to evaluate their effect on the ensuing \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics.
The possible role of an elevated metabolic rate, dissociated from on \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics, has been investigated. In one study, for instance, the investigators showed that the dynamic response of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\), and hence of muscular aerobic metabolism, is decelerated when the exercise is resumed starting, during recovery, from a raised metabolic rate (Bowen et al. 2011). This finding was attributed to the recovering muscle's less-favorable energetic status, which directly caused a longer delay of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) in attaining the steady state.
The present investigation was undertaken to challenge these two opposing hypotheses regarding the behavior of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics when the exercise is resumed after different recovery times. The first one predicts that smaller values of starting [PCr] before the onset of the exercise are related to a progressive acceleration of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics. The opposite hypothesis states that the raised metabolic rate and the disadvantageous energetic state of the muscle may be associated with a slower \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics. To this aim, we studied in exercising humans the effects of different recovery times on \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics during a square-wave cycling exercise transition performed at the same mechanical power. By manipulating the time of recovery, we induced conditions characterized by metabolic rates and [PCr], which are in opposition: for short recovery times, we have high metabolic rates and low [PCr]; the opposite is true for longer times of recovery. In addition, the results may also allow us to evaluate whether the τ2 of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetic (from now on, we refer simply to “τ”) is linearly related with the estimated ∆PCr occurring during the transients or not.
Methods
Subjects
Seven healthy, young men were investigated (age: 23.0 ± 2.3 years; height: 1.76 ± 0.06 m, body mass: 69.8 ± 3.6 kg; \(\dot{V}{\text{O}}_{{2{\text{peak}}}}\): 3.32 ± 0.67 L min−1; \(\dot{V}{\text{O}}_{{2{\text{peak}}}}\) per kg: 47.7 ± 10.7 ml kg−1 min−1). All the subjects were engaged in regular physical activities. The participants were informed about the procedures and risks associated with the experimental design and protocol, and they signed an informed consent form before participating in the study. The institutional review board of the Department of Neurological and Movement Sciences, University of Verona, approved the study protocol, the experimental design, and methods that conformed to the 1964 Declaration of Helsinki.
Protocol
The experiments were carried out on the same day of 7 subsequent weeks at the Exercise Physiology Laboratory of the Department of Neuroscience, Biomedicine and Movement Sciences, University of Verona. The subjects were asked to sleep at least 8 h, abstain from vigorous physical activity in the 24 h preceding each test, and consume a light meal 2–3 h before reaching the laboratory. All the tests were performed at approximately the same time of the day under controlled environmental conditions (22–25 °C, 55–65% relative humidity).
On the first day, anthropometric measurements were taken. Then, the subjects were familiarized with the tasks and performed a maximal incremental ramp test to determine \(\dot{V}{\text{O}}_{{2{\text{peak}}}}\) and gas-exchange threshold. The cycle ergometer seat and handlebar positions were customized for each subject and then maintained for all subsequent tests in this session.
The ramp test consisted of 3 min at rest, 5 min of warm-up exercise at 50 W, followed by a continuous increase of the workload by 20 W per minute until voluntary exhaustion. The accepted criteria for maximal effort were: respiratory exchange ratio > 1.1 and heart rate (HR) > 90% of the predicted maximum based on age. At the end of the test, blood lactate concentration ([La]b) was measured in arterialized capillary blood obtained from an ear lobe (20 μl).
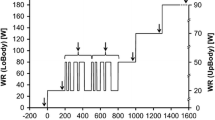
The constant-load exercise tests were carried out in the following six experimental sessions and consisted of two series of square-wave cycling exercises performed at a workload of 120 W. Each exercise phase lasted 6 min and was interspersed with different times of recovery of 30 s, 60 s, 90 s, 120 s, and 300 s performed at unloaded pedaling maintaining the same RPM used during loaded exercise. Two symmetric sequences (protocol A and protocol B) were devised, and they were administrated randomly to the volunteer in each session to avoid habituation effects (Fig. 1). Each sequence was repeated on different days three times. The first transition was always preceded by a 5-min phase of data collection at rest followed by 3 min of unloaded pedaling. The first constant-load exercise transition (C in Fig. 1) was always followed by a recovery phase of 300 s, during which [La]b was measured at the 1st, 3rd, and 5th min of recovery to assess peak [La]b.
Schematic representation of the applied protocols. In protocol A, after a square transition of 6 min followed by 5 min of recovery, a series of five identical transitions followed. Each exercise phase was separated by increasing times of recovery from 30 to 120 s. In protocol B, the order of the times of recovery was reverted
Methods
\(\dot{V}{\text{O}}_{{2{\text{A}}}}\) was measured using a metabolic cart (Quark B2, Cosmed, Italy). Subjects wore a nose clip and breathed through a mouthpiece mounted on a turbine flow meter. Gases were sampled continuously through a capillary line inserted in the outer frame of the flow meter and analyzed by fast-response O2 (chemical) and CO2 (infrared) sensors embedded in the metabolic cart. The metabolic cart software allowed us to record gas and flow signals (sampling frequency: 25 Hz) and save them as ASCII files. The analyzers and the propeller were calibrated before each experimental run: (i) with a gas mixture of known composition (FO2 = 0.16; FCO2 = 0.04; N2 as balance) and ambient air; (ii) using a 3 L syringe (Hans Rudolph Inc., USA), following the procedures indicated by the manufacturer. The electromagnetically braked cycle ergometer (SportExcalibur, Lode, D) was connected to and operated by a PC running the metabolic cart. The system made it possible to impose work rates according to pre-defined exercise protocols. The electromechanical characteristics of the ergometer permitted an almost instantaneous step change from one work rate to the next (the response time of the ergometer was around 80 ms, according to the operating manual and as measured in a preliminary session).
[La]b, was assessed using an electro-enzymatic method (Biosen C_line, EKF Diagnostics, Barleben, Germany).
Data analysis. Single breath alveolar oxygen uptake (\(\dot{V}{\text{O}}_{{2{\text{A}}}}\)) was then calculated from the original gas and flow traces utilizing an algorithm (Grønlund 1984) that considers variations of the lung’s O2 stores that occur from one breath to another. In particular, the N2 and Ar concentration was estimated as 100% minus the sum of measured O2 and CO2 concentrations, as also utilized by Clemensen and colleagues (Clemensen et al. 1994). The algorithm was implemented using an automated procedure written in the object-oriented G language, implemented in the developing environment Labview 5.0 (National Instruments, USA) and running on a PC (HP, USA) (Cautero et al. 2003).
GET was individually estimated by applying the Wasserman method (Wasserman et al. 1999). \(\dot{V}{\text{O}}_{{2{\text{peak}}}}\) and all maximal variables were calculated as the average of the last 30 s before the end of the exercise.
The \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) time series were aligned with the onset of the first work rate transition of protocols A and B and treated by subtracting the average \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) prevailing during the last 15 s preceding the workload change, except for C and C1 where the averages were calculated on the data of the last minute of the phase preceding exercise. Each of the three sequences was then interpolated to a 1-s interval, according to Lamarra et al. (1987), and averaged to get a single data file for each evaluated workload transition in each subject. Finally, each ON-phase of the different square transitions of protocols A and B was separated, plotted as a function of time from 0 to 300 s, and interpolated utilizing a mono-exponential model with a time delay
where: Y(t): \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) at time t; A (L min−1) = amplitude of the \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) response; TD(s) is the time delay of the response; τ(s) is the time constant of the mono-exponential function.
The parameters of the model [i.e., amplitude (A), time constant (τ), time delay, TD] were estimated using a weighted non-linear least-squares fitting procedure implemented with Sigmaplot 11 (Systat Software San Jose, CA), as described previously (De Roia et al. 2012). Equation 1 is true only for t > TD and it was fitted without considering the \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) data of the first 20 s of each transition. Functional gain (G, in ml min−1 W−1) was calculated as the ratio between \(\dot{V}{\text{O}}_{2}\) at steady state and ∆WR, where WR is the work rate.
Statistics
Values are always presented as mean plus/minus the corresponding standard deviations. Confidence intervals of the estimated τ were calculated from the asymptotic standard errors of the parameters (Motulsky and Christopoulos 2004). Differences between the τ values obtained from the \(\dot{V}{\text{O}}_{{2{\text{A}}}}\)-ON kinetics investigated at different recovery times were evaluated using a one-way ANOVA for repeated measures with the recovery time as the within-subjects factor. The sphericity of the pooled data was assessed employing the epsilon statistics. When epsilon was significantly different from 1, the Geisser/Greenhouse correction for the degrees of freedom was applied. Post hoc analysis for the influence of the recovery time on the τ of the \(\dot{V}{\text{O}}_{{2{\text{A}}}}\)-ON kinetics was performed using pairwise comparison using the Tukey multiple comparison test. Linear regressions were calculated utilizing the least-square method, and the corresponding coefficients of determination were obtained. α was set to 0.05. The parameters of the non-linear mono-exponential decay of τ as a function of recovery time were estimated using weighted non-linear least-squares procedure (Marquardt, 1963). Analysis was performed using the GraphPad Prism version 9.0.1 for macOS (San Diego, CAS, USA).
Results
The \(\dot{V}{\text{O}}_{2}\) corresponding to GET amounted, on average, to 30.5 ± 10.7 ml kg−1 min−1 and to 47 ± 9.6% of the individual \(\dot{V}{\text{O}}_{{2{\text{peak}}}}\). Mechanical power at GET was 159 ± 41. 4 W. Peak [La]b after the C square-wave exercise amounted to 2.7 mM ± 0.07 and peak lactate accumulation was 1.7 mM ± 0.07.
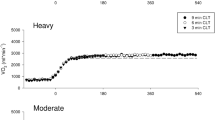
In Fig. 2, breath-by-breath \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) values of a typical subject are reported, after superposition, and synchronization, as a function of time from exercise onset during square-wave transitions performed at the same absolute workload but preceded by variable recovery intervals. The control condition C always started from rest, whereas C1 was preceded by 300 s of recovery. The graphs also report the corresponding mono-exponential fitting functions and the estimated τ.
Exercise transitions after the different recovery times started from different metabolic rates. After 30 s of recovery \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) was 1.32 l min−1 ± 0.13; after 60 s, it amounted to 0.89 l min−1 ± 0.13; at 90 s of recovery the subjects resumed exercise when \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) corresponded to 0.66 l min−1 ± 0.12; and after 120 s, \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) was 0.58 l min−1 ± 0.95. In addition, \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) values prevailing before C (0.44 l min−1 ± 0.06) and C1 (0.57 l min−1 ± 0.03) were significantly different (P < 0.01; 95% CI of difference: 0.10–0.17 l min−1).
In Table 1, the average values of the functional gains (G), amplitudes (A), time constants (τ), and time delays (TD) of the \(\dot{V}{\text{O}}_{{2{\text{A}}}}\)-ON kinetics corresponding to the different recovery times are presented together with their standard deviations and the 95% confidence limits of the estimated τ.
Amplitudes of the responses were significantly different in the various conditions. In detail,
-
amplitude of C transition was significantly larger than at C1 (P = 0.020), 30 s (P < 0.001), 60 s (P < 0.001), 90 s (P < 0.001), and 120 s (P = 0.009);
-
amplitude of C1 transition was larger than at 30 s (P < 0.001) and 60 s (P = 0.003);
-
amplitude of 30 s transition was smaller than at 60 s (P < 0.001), 90 s (P < 0.001), and 120 s (P < 0.001);
-
amplitude of 60 s transition was smaller than at 90 s (P < 0.001) and 120 s (P < 0.001);
-
amplitude of 90 s transition was smaller (P = 0.008) than at 120 s.
Only the amplitudes after 90 s and 120 s of recovery were not significantly different from those assessed after 300 s of recovery (C1).
However, functional gains (grand average 12.1 ml min−1 W−1 ± 0.15), calculated as the ratio between \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) at steady state and workload, and \(\dot{V}{\text{O}}_{{2{\text{ss}}}}\) (grand average: 1.96 l min-1 ± 0.02) were not significantly different for the different times of recovery. Time delays were not affected by the recovery time in any of the cases.
τ of the step transition preceded by the shortest time of recovery (30 s) was significantly larger than the ones of the transitions corresponding to longer recovery times (60 s: P = 0.035, 95% CI of difference: 0.62–14.6 s; 90 s: P = 0.022, 95% CI of difference: 1.67–18.53 s). τ of the transition after the shortest time of recovery (30 s) tended to be larger than the one assessed during exercise performed after 120 s of recovery (P = 0.0905). Likewise, τ assessed after 1 min of recovery tended to be larger than the one estimated during the step transition imposed after 90 s of recovery (P = 0.063). Finally, the τ of the \(\dot{V}{\text{O}}_{{2{\text{A}}}}\)-ON response in C was significantly longer than in C1 (P = 0.044). The decay of τ seemed to decay as a first-order exponential decay of the time of recovery from 30 to 300 s: τ = 109.5 × e(−t/14.0) + 18.9; r2 = 0.32) (Fig. 3).
Discussion
The results obtained in this investigation showed that the τ of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\)-ON kinetics of a square-wave transition imposed after different recovery times at moderate-intensity exercise decreased with recovery time. Since the amount of PCr resynthesized during recovery is proportional to the volume of O2 consumed during the rapid phase of the O2 debt repayment, this would also imply that τ might be affected by the [PCr] existing at the end of recovery or by ∆[PCr] occurring during the subsequent transient. Alternatively, it may be influenced by the raised metabolic rate prevailing before exercise resumption and, hence, on the energetic status of the muscles.
Since τ of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\)-ON kinetics is a close proxy of τPCr, i.e., of the τ of PCr splitting at the onset of exercise, we may speculate that the starting [PCr] and/or ∆[PCr] influence, in the same fashion, also the splitting rate of PCr during the transient.
At variance with other investigations where muscular [PCr] and ∆[PCr] were directly assessed during step exercise transitions (f.i., Francescato et al. 2008), in the present study, muscular [PCr] and ∆[PCr] were not directly measured. Therefore, the paragraphs here below are devoted to discussing the current findings in the light of directly obtained data related to the muscular metabolic condition.
\(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics and priming exercise
\(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics in the C1 condition, i.e., when exercise was preceded by complete recovery [PCr] after a series of square-wave transitions of moderate-intensity exercise, was significantly faster than when the same workload change was not preceded by any priming exercise (C). In addition, the two amplitudes were significantly different, because C1 initiated from a higher oxygen uptake at rest than in C.
The acceleration of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics is a common finding (DeLorey et al. 2004; Gerbino et al. 1996; Gurd et al. 2005; Zoladz et al. 2006) when we resume exercise after priming, heavy-intensity exercise. The acceleration of the kinetics has been commonly attributed to better matching between local O2 delivery and consumption (De Roia et al. 2012), to a faster response of bulk, cardiovascular O2 delivery (Gerbino et al. 1996), or a quick activation of pyruvate dehydrogenase after a bout of exercise (Gurd et al. 2006). In this case, even moderate-intensity exercise elicited a substantial acceleration of the \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics in the subsequent transitions. This unexpected result may be the consequence of a not particularly high level of physical fitness of our volunteers, who, even for a moderate-intensity exercise, could benefit from the priming effect on \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics in the subsequent transition.
\(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics and PCr concentration
As we outlined in the introduction, two models of mitochondrial respiratory control were proposed to explain the link between the splitting rate of muscular PCr during rest-to-work transients and: (i) the split amount of PCr during transient (∆PCr) or (ii) the [PCr] prevailing before the transition.
According to one model, on one hand, the τ of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics is determined by ∆PCr, so that \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics accelerates as ∆PCr decreases (Korzeniewski and Zoladz 2004). On the other hand, data obtained using 31P-NMR spectroscopy suggested that the τ of PCr splitting during transitions starting from rest is linearly related to resting [PCr] (Francescato et al. 2008). These findings imply that [PCr] itself is one of the main controllers of oxidative phosphorylation in the skeletal muscle (Walsh et al. 2001). According to the investigators, resting [PCr] would act as an energy buffer reserve whose value would be inversely related to the speed of the dynamic response of oxidative metabolism at the beginning of the exercise: the larger the store, the slower the on-kinetics of oxidative metabolism. These findings are also consistent with those obtained in rats fed with a creatine analog that reduced [Cr] and [PCr] contents at rest and with the hypothesis that the creatine kinase reaction acts as a chemical capacitor leading to a value of the τ of PCr splitting proportional to the initial [Cr] (Meyer 1989). It is also consistent with a linear model of respiration control in skeletal muscle (Walsh et al. 2001).
Therefore, it is tempting to discuss the present data based on these theories and facts.
In this investigation, muscular [PCr] and ∆[PCr] were not directly measured. Instead, only DefO2 could be calculated based on breath-by-breath \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) and [PCr] prevailing at the end of the recovery and ∆[PCr] can be therefore tentatively estimated based on: (i) the calculated DefO2 and; (ii) some reasonable assumptions concerning the mass of muscles recruited during exercise and muscular [PCr] at rest.
DefO2 contracted at the onset of each square-wave transition was first calculated as the difference between: (1) the volume of O2 that one should have consumed if \(\dot{V}{\text{O}}_{{2{\text{ss}}}}\) had been attained immediately at the beginning of the exercise minus (2) the volume of O2 taken up during the exercise. The first member of the difference was calculated by multiplying the difference between (1) \(\dot{V}{\text{O}}_{{2{\text{ss}}}}\) and (2) \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) at the end of the preceding recovery phase for the duration of the corresponding trial. The second term, i.e., the volume of O2 consumed during exercise, was calculated by summing progressively net \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) from the onset of exercise to the end of the trial. Afterward, the DefO2 in ml of O2 was converted in mmol of O2 dividing it by 22.4 ml mmol O2−1. The amount of PCr split during the transient was then calculated by multiplying DefO2 (mmol of O2) times the P/O2 ratio adjusted for the respiratory exchange ratio calculated at steady state (P/O2 = 4.13 + 2.07 RER) (di Prampero 1981). To calculate the corresponding ∆[PCr], the absolute value of PCr obtained at the end of the calculations was divided by one-third of the individual body mass, assuming that during cycling, the subjects recruited a muscle mass equivalent to 33% of total body mass. [PCr] at steady state was finally obtained from the difference between [PCr] at rest, assumed equal to 25 mmol kg−1 of fresh muscle, and that obtained from DefO2. The volume of O2 taken up during each recovery phase, the absolute amount of resynthesized PCr, and the corresponding increase of muscular [PCr] were obtained applying similar calculations. Once added to the [PCr] prevailing at steady state, the last value yielded the [PCr] existing at the end of each recovery phase and, hence, before the subsequent square-wave transition.
In Fig. 4, the estimated [PCr] and ∆[PCr] are plotted as a function of the time of recovery preceding the exercise transitions. Both quantities increased as a function of a time of recovery up to 120 s ([PCr] = 0.094 time + 14.9, r2 = 0.979; ∆[PCr] = 0.021 time + 3.33, r2 = 0.94). The τ of the primary phase of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics turned out to be a decreasing function of the [PCr] (τ = − 1.07 [PCr] + 44.9, r2 = 0.513, P < 0.01; F = 34.8). Conversely, it seemed not to be affected by ∆[PCr] (τ = 0.096 ∆[PCr] + 20.96, r2 = 0.001, F = 0.033). Given these results, one may also expect that the τ \(\dot{V}{\text{O}}_{2}\) on-kinetics depends on the recovery time according to a first-order decaying function (Vinetti et al. 2017), as it is suggested in Fig. 3.
A Estimated [PCr] (full circles) and ∆[PCr] (open circles) prevailing before the onset of square-wave exercise as a function of the time of recovery. Big circles (at time of recovery of 300 s) refer to the control conditions. [PCr] at rest and after 300 s of recovery was assumed equal to 25 mmol kg−1 of fresh muscle; ∆[PCr] values at rest and after a complete recovery were used to obtain a single mean value: B τ as a function of [PCr] prevailing at the end of recovery before the onset square-wave transitions; C τ as a function of ∆[PCr] consumed during the transient phase of each square-wave transitions
Therefore, these present calculations yield results that contrast with the experimental (Francescato et al. 2008) and predicted data (Korzeniewski and Zoladz 2004) and also with the predictions according to the model of Meyer (1988)
\(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics and energetic muscle state
The obtained data, although in contrast with those predicted (Korzeniewski and Zoladz 2004) or showed (Francescato et al. 2008), agree with the findings presented by other investigators who showed that the τ of the primary phase is significantly longer when the square-wave transient is imposed after 20 s of recovery following the abrupt interruption of moderate-intensity, constant rate exercise, so that exercise is resumed from an increased level of the metabolism (Bowen et al. 2011).
Several biochemical/enzymatic and thermodynamic modifications may explain the altered \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) of exercise starting from an elevated baseline metabolic rate. It has been proposed that mitochondrial oxidative rate conforms to Michaelis–Menten enzyme kinetics via the substrates involved in ATP resynthesis (Chance et al. 1981). In this case, [ADP] and [Pi] would influence the rate of increase of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\). For instance, Km for ADP depends upon [ATP] and [Pi] (Wilson 1994). Therefore, when metabolic rate and mitochondrial respiration are elevated, the signal deriving from the phosphorylation potential ([ATP]/ [ADP] × [Pi]) should be more prominent to elicit a given increase of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\). This speculation is further strengthened by the findings that oxidative phosphorylation is not linearly related to the free energy of ATP hydrolysis (∆GATP) which is, in turn, affected by metabolite concentrations. This matter of fact would imply that more significant changes in [ADP] and [Pi] would have been required for each increase of oxidative phosphorylation at elevated metabolic rates (Jeneson et al. 1995).
The reduced value of the starting ∆GATP at the onset of exercise would require a greater ATP synthesis. This mechanism would also predict a higher \(\dot{V}{\text{O}}_{{2{\text{ss}}}}\) (and a more extensive functional gain, i.e., the net increment of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) per unit of workload increase) for the same absolute workload. This in turn implies that \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) would take more time to attain \(\dot{V}{\text{O}}_{{2{\text{ss}}}}\) with the progressive fall of ∆GATP as suggested by others (Bowen et al. 2011). However, this mechanism cannot be advocated as the possible explanation of the observed findings in the present investigation, since neither \(\dot{V}{\text{O}}_{{2{\text{ss}}}}\) nor gains were affected by recovery time.
In contrast with the results obtained in this investigation, on one hand, starting from an elevated baseline metabolic rate did not decelerate the \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics during bouts of high-intensity exercise initiated from a \(\dot{V}{\text{O}}_{2}\) level identical to \(\dot{V}{\text{O}}_{{2{\text{ss}}}}\) of moderate-intensity exercise (DiMenna et al. 2010). On the other hand, the τ of the \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics investigated during high-intensity exercise transitions performed from unloaded pedaling was significantly longer than the present results (DiMenna et al. 2010). The slowed \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics may be the consequence of the more extensive recruitment of fast motor units at higher work rates than occurring at the onset of moderate-intensity exercise, as in the present case. The motor units progressively recruited at higher work rates are formed by IIX fibers characterized by lower mitochondrial content and P/O ratio, longer PCr splitting kinetics, and higher [PCr], and moreover, they rely more on the anaerobic glycolytic production of ATP. All these features have been directly related to a slower \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics (Korzeniewski and Zoladz 2004). For all these reasons, the results suggesting a slower response of the oxidative energy-yielding pathway obtained during rest-to high-intensity exercise transition cannot be directly compared with those obtained in the present investigation. They should be likely attributed to different neuromuscular, cellular, and biochemical mechanisms.
\(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics and circulatory dynamics
The results presented by Francescato and colleagues (Francescato et al. 2008) concern the τ of PCr splitting were obtained investigating the time course of PCr disappearance and synthesis using magnetic resonance spectroscopy applied on small muscle groups.
Our experiments were carried out in vivo on exercising humans. They considered only alveolar breath-by-breath O2 transfer and estimated the corresponding [PCr] absolute concentrations and changes. At the sudden onset of a constant-load exercise, \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) conforms to a two-component time course whose first small amplitude and rapid phase are mainly dictated by the rapid increase of pulmonary blood flow. Only the second one (metabolic or primary phase) is a reliable proxy of muscular O2 uptake. However, as it has been appropriately pointed out that (Francescato et al. 2013), between the muscle, i.e., the final utilization site of O2, and the lung, where O2 uptake is measured, at least two buffers exist. They are the cardiovascular system, since the dynamic response of convective O2 transport may distort alveolar O2 uptake and the interposition of the amount of O2 bound, before exercise transient, to the O2 venous stores. The effect of the O2 stored inside the body before the exercise transient, and already available to the muscles, consists in slowing down \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics, as the latter measures only the volume of O2 taken up from the environment and transferred across the alveolar-capillary membrane. Thus, on purely theoretical grounds, the slower alveolar \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics might be attributed, ceteris paribus, to different absolute values of O2 venous stores prevailing at the end of each period of recovery and/or to a kinetic dissociation between the rate of O2 muscular uptake and the cardiovascular dynamics in the different evaluated recovery conditions. However, it has also been demonstrated with in silico computational simulation that the high \(\dot{Q}/\dot{V}{\text{O}}_{{2{\text{m}}}}\) prevailing in the condition of the shortest recovery time (30 s) would elicit the decrease of τ − \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) (Bowen et al. 2011). Therefore, the deceleration of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics observed in coincidence with the shortest recovery time should likely be of muscular origin.
In summary, the results obtained in this investigation do not agree with the view that a decreased pre-exercise [PCr] brings about a substantial acceleration of aerobic metabolism in the muscle whose dynamic adjustments are described in exercising humans by \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics. Instead, \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) kinetics seems to be influenced by the pre-transition metabolic rate, implying that the request of ATP mitochondrial production rises in proportion to the metabolism of the muscle and the consequent changes of intracellular energetic status.
Abbreviations
- A :
-
Amplitude of the mono-exponential response
- ATP:
-
Adenosine triphosphate
- [ATP]:
-
Adenosine triphosphate concentration
- [ADP]:
-
Adenosine diphosphate concentration
- CI95:
-
95% Confidence intervals
- ∆G ATP :
-
Free energy of ATP hydrolysis
- ∆[PCr]:
-
Amount of PCr split during rest-to-work transition
- DefO2 :
-
Oxygen deficit
- G :
-
Functional gain of \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) at steady state
- GET:
-
Gas exchange threshold
- HR:
-
Heart rate
- [La]b :
-
Blood lactate concentration
- [Pi]:
-
Inorganic muscle phosphate concentration
- [PCr]:
-
Phosphocreatine concentration
- τ (tau):
-
Time constant
- τ [PCr]:
-
Time constant of phosphocreatine splitting kinetic
- τ 1 :
-
Time constant of (Phase 1, or cardio-dynamic phase) of alveolar O2 kinetic
- τ 2 :
-
Time constant of (Phase 2, or primary phase) of alveolar O2 kinetic
- \(\dot{V}{\text{O}}_{2}\) :
-
Oxygen consumption
- \(\dot{V}{\text{O}}_{{2{\text{A}}}}\) :
-
Alveolar oxygen uptake
- \(\dot{V}{\text{O}}_{{2{\text{m}}}}\) :
-
Uptake at muscles levels
- \(\dot{V}{\text{O}}_{{2{\text{ss}}}}\) :
-
Steady-state oxygen uptake
- \(\dot{V}{\text{O}}_{{2{\text{peak}}}}\) :
-
Peak oxygen uptake
- WR:
-
Work rate
References
Bowen ST, Murgatroyd SR, Cannon DT, Cuff TJ, Lainey AF, Marjerrison AD, Spencer MD, Benson AP, Paterson DH, Kowalchuk JM, Rossiter HB (2011) A raised metabolic rate slows pulmonary O2 uptake kinetics on transition to moderate-intensity exercise in humans independently of work rate. Exp Physiol 96:1049–1061
Cautero M, di Prampero PE, Capelli C (2003) New acquisitions in the assessment of breath-by-breath alveolar gas transfer in humans. Eur J Appl Physiol 90:231–241
Cautero M, di Prampero PE, Tam E, Capelli C (2005) Alveolar oxygen uptake kinetics with step, impulse and ramp exercise in humans. Eur J Appl Physiol 95:474–485
Chance B, Eleff S, Leigh JS Jr, Sokolow D, Sapega A (1981) Mitochondrial regulation of phosphocreatine/inorganic phosphate ratios in exercising human muscle: a gated 31P NMR study. Proc Natl Acad Sci USA 78:6714–6718
Clemensen P, Christensen P, Norsk P, Grønlund L (1994) A modified photo and magnetoacustic multigas analyzer applied in gas exchange measurements. J Appl Physiol 76:2832–2839
De Roia G, Pogliaghi S, Adami A, Papadopoulou C, Capelli C (2012) Effects of priming exercise on the speed of adjustment of muscle oxidative metabolism at the onset of moderate-intensity step transitions in older adults. Am J Physiol Regul Integr Comp Physiol 302:R1158–R1166
DeLorey DS, Kowalchuk JM, Paterson DH (2004) Effects of prior heavy intensity exercise on pulmonary O2 uptake and muscle deoxygenation kinetics in young and older adult humans. J Appl Physiol 97:998–1005
di Prampero PE (1981) Energetics of muscular exercise. Rev Physiol Biochem Pharmacol 89:143–222
DiMenna FJ, Bailey SJ, Vanhatalo A, Chidnok W, Jones AM (2010) Elevated baseline [Inline Image Removed]O2 per se does not slow V̇O2 uptake kinetics during work-to-work exercise transitions. J Appl Physiol 109:1148–1154
Ferretti G, Fagoni N, Taboni A, Vinetti G, di Prampero PE (2022) A century of exercise physiology: key concepts on coupling respiratory oxygen flow to muscle energy demand during exercise. Eur J Appl Physiol 122(6):1317–1365. https://doi.org/10.1007/s00421-022-04901-x
Francescato MP, Cettolo V, di Prampero PE (2008) Influence of phosphagen concentration on phosphocreatine breakdown kinetics. Data from human gastrocnemius muscle. J Appl Physiol 105:158–164
Francescato MP, Cettolo V, di Prampero PE (2013) Oxygen uptake kinetics at work onset: Role of cardiac output and of phosphocreatine breakdown. Resp Physiol Neurobiol 185:287–295
Gerbino A, Ward SA, Whipp BJ (1996) Effects of prior exercise on pulmonary gas-exchange kinetics during high-intensity exercise in humans. J Appl Physiol 80:99–107
Grassi B (2003) Oxygen uptake kinetics: old and recent lessons from experiments on isolated muscle in situ. Eur J Appl Physiol 90:242–249
Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD (1996) Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol 80:988–998
Grassi B, Gladden B, Stary CM, Wagner PD, Hogan MC (1998) Peripheral O2 diffusion does not affect VO2 on-kinetics in isolated in sit canine muscle. J Appl Physiol 85:1404–1412
Greiner A, Esterhammer R, Bammer D, Messner H, Kremser C, Jaschke WR, Fraedrich G, Schocke MFH (2007) High-energy phosphate metabolism in the calf muscle of healthy humans during incremental calf exercise with and without moderate cuff stenosis. Eur J Appl Physiol 99:519–531
Grønlund L (1984) A new method for breath-to-breath determination of oxygen flux across the alveolar membrane. Eur J Appl Physiol 52:167–172
Gurd BJ, Scheuermann BW, Paterson DH, Kowalchuk JM (2005) Prior heavy-intensity exercise speeds [Inline Image Removed]O2 kinetics during moderate-intensity exercise in young adults. J Appl Physiol 98:1371–1378
Gurd BJ, Peters SJ, Heigenhauser GJ, LeBlanc PJ, Doherty TJ, Paterson DH, Kowalchuk JM (2006) Prior heavy exercise elevates pyruvate dehydrogenase activity and speeds O2 uptake kinetics during subsequent moderate-intensity exercise in healthy young adults. J Physiol 577:985–996
Haseler LJ, Kindig CA, Richardson RS, Hogan MC (2004) The role of oxygen in determining phosphocreatine onset kinetics in exercising humans. J Physiol 558:985–992
Jeneson JA, Westerhoff HV, Brown TR, Van Echteld CJ, Berger R (1995) Quasi-linear relationship between Gibbs free energy of ATP hydrolysis and power output in human forearm muscle. Am J Physiol Cell Physiol 268:C1474-1484
Kindig CA, Howlett RA, Stary CM, Walsh B, Hogan MC (2005) Effects of acute creatine kinase inhibition on metabolism and tension development in isolated single myocytes. J Appl Physiol 98:541–549
Korzeniewski B, Zoladz JA (2001) A model of oxidative phosphorylation in mammalian skeletal muscle. Biophys Chem 92:17–34
Korzeniewski B, Zoladz JA (2004) Factors determining the oxygen consumption rate ( O2) on-kinetics in skeletal muscles. Biochem J 379:703–710
Lador F, Azabji Kenfack M, Moia C, Cautero M, Morel DR, Capelli C, Ferretti G (2006) Simultaneous determination of the kinetics of cardiac output, systemic O2 delivery, and lung O2 uptake at exercise onset in men. Am J Physiol Regul Integr Comp Physiol 290:R1071–R1079
Lamarra N, Whipp BJ, Ward SA, Wasserman K (1987) Effect of interbreath fluctuations on characterising exercise gas exchange kinetics. J Appl Physiol 62:2003–2012
Loeppke JA, Greene ER, Hoekenga DE, Caprihan A (1981) Beat by-beat stroke volume assessment by pulsed Doppler in upright and supine exercise. J Appl Physiol 50:1173–1182
Marquardt DW (1963) An algorithm for least – squares estimation of nonlinear parameters. J Soc Ind Appl Math 11:431–444
McCreary CR, Chilibeck PD, Marsh GD, Paterson DH, Cunningham DA, Thompson RT (1996) Kinetics of pulmonary oxygen uptake and muscle phosphates during moderate-intensity calf exercise. J Appl Physiol 81:1331–1338
Meyer RA (1988) A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol Cell Physiol 254:C548–C553
Meyer RA (1989) Linear dependence of muscle phosphocreatine kinetics on total creatine content. Am J Physiol Cell Physiol 257:C1149–C1157
Motulsky H, Christopoulos A (2004) Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting. Oxford University Press
Piiper J, Spiller P (1970) Repayment of O2 debt and resynthesis of high-energy phosphates in gastrocnemius muscle of the dog. Am J Physiol 28:657–662
Piiper J, di Prampero PE, Cerretelli P (1968) Oxygen debt and high-energy phosphates in gastrocnemius muscle of the dog. Am J Physiol 215:523–531
Poole DC, Jones AM (2005) Towards an understanding of the mechanistic bases of V̇O2 kinetics. In: Jones AM, Poole DC (eds) Oxygen uptake kinetics in sport, exercise and medicine. Routledge, pp 294–328
Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JR, Whipp BJ (1999) Inferences from O2 pulmonary uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol 518:921–932
Vinetti G, FagonoiN TA, Camelio S, di Prampero PE, Ferretti G (2017) Effects of recovery interval duration on teh parameters of the critical power model for incremental exercise. Eur J Appl Physiol 117:1859–1867
Walsh B, Tonkonogi M, Söderlund K, Hultman E, Saks V, Sahlin K (2001) The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol 537:971–978
Wasserman K, Hansen JE, Sue DY, Casaburi R, Whipp BJ (1999) Principles of exercise testing and interpretation, 3rd edn. Lippincott Williams & Wilkins, Baltimore
Whipp BJ, Ward SA (1990) Physiological determinants of pulmonary gas exchange kinetics during exercise. Med Sci Sports Exer 22:62–71
Whipp BJ, Rossiter HB, Ward SA, Avery D, Doyle VL, Howe FA, Griffiths JR (1999) Simultaneous determination of muscle 31P and O2 uptake kinetics during whole body NMR spectroscopy. J Appl Physiol 86:742–747
Wilson DF (1994) Factors affecting the rate and energetics of mitochondrial oxidative phosphorylation. Med Sci Sports Exerc 26:37–43
Yoshida T, Yamamoto K, Udo M (1993) Relationship between cardiac output and oxygen uptake at the onset of exercise. Eur J Appl Physiol 66:155–160
Zoladz JA, Korzeniewski B, Grassi B (2006) Training-induced acceleration of oxygen uptake kinetics in skeletal muscle: the underlying mechanisms. J Physiol Pharmacol 57(S10):67–84
Acknowledgements
The authors greatly acknowledge the help of the volunteers without whom this study would not have been possible.
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Guido Ferretti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pogliaghi, S., Tam, E. & Capelli, C. Effect of recovery time on \(\dot{V}{\text{O}}_{2}\)-ON kinetics in humans at the onset of moderate-intensity cycling exercise. Eur J Appl Physiol 123, 261–270 (2023). https://doi.org/10.1007/s00421-022-05057-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-022-05057-4