Abstract
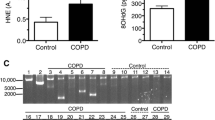
Chronic obstructive pulmonary disease (COPD) is frequently associated with age-related muscle loss or sarcopenia. However, the exact molecular mechanism of muscle loss in COPD remains elusive. We investigated the association of chronic dysregulation of sarcoplasmic reticulum (SR) protein homeostasis (a condition called SR stress) and myonuclear disorganization with sarcopenia in patients with COPD. Markers of SR stress and their downstream consequences, including apoptosis and inflammation, were upregulated in patients with COPD. The maximal SR Ca2+ ATPase (SERCA) activity was significantly reduced in advanced COPD as compared to healthy controls. Single muscle fiber diameter and cytoplasmic domain per myonucleus were significantly smaller in patients with advanced COPD than in healthy controls. Increased disruption of myonuclear organization was found in the COPD patients as compared to healthy controls. These changes in SR dysfunction were accompanied by elevated global levels of oxidative stress, including lipid peroxidation and mitochondrial reactive oxygen species (ROS) production. Altogether, our data suggest that muscle weakness in advanced COPD is in part associated with the disruption of SR protein and calcium homeostasis and their pathological consequences.






Similar content being viewed by others
Data availability
Data are available from the corresponding author on request.
References
Afroze D, Kumar A (2019) ER stress in skeletal muscle remodeling and myopathies. FEBS J 286(2):379–398
Agusti AG, Sauleda J, Miralles C, Gomez C, Togores B, Sala E, Batle S, Busquets X (2002) Skeletal muscle apoptosis and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166(4):485–489
Alamdari N, Toraldo G, Aversa Z, Smith I, Castillero E, Renaud G, Qaisar R, Larsson L, Jasuja R, Hasselgren PO (2012) Loss of muscle strength during sepsis is in part regulated by glucocorticoids and is associated with reduced muscle fiber stiffness. Am J Physiol Regul Integr Comp Physiol 303(10):R1090-1099
Alibegovic AC, Sonne MP, Hojbjerre L, Bork-Jensen J, Jacobsen S, Nilsson E, Faerch K, Hiscock N, Mortensen B, Friedrichsen M, Stallknecht B, Dela F, Vaag A (2010) Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am J Physiol Endocrinol Metab 299(5):E752-763
Bahar E, Kim H, Yoon H (2016) ER stress-mediated signaling: action potential and Ca(2+) as key players. Int J Mol Sci 17(9):1558
Barreiro E, Jaitovich A (2018) Muscle atrophy in chronic obstructive pulmonary disease: molecular basis and potential therapeutic targets. J Thorac Dis 10(Suppl 12):S1415–S1424
Barreiro E, Salazar-Degracia A, Sancho-Munoz A, Aguilo R, Rodriguez-Fuster A, Gea J (2019) Endoplasmic reticulum stress and unfolded protein response in diaphragm muscle dysfunction of patients with stable chronic obstructive pulmonary disease. J Appl Physiol 126(6):1572–1586
Bartoli M, Richard I (2005) Calpains in muscle wasting. Int J Biochem Cell Biol 37(10):2115–2133
Berton E, Antonucci R, Palange P (2001) Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Monaldi Arch Chest Dis 56(5):418–422
Bhaskaran S, Unnikrishnan A, Ranjit R, Qaisar R, Pharaoh G, Matyi S, Kinter M, Deepa SS (2017) A fish oil diet induces mitochondrial uncoupling and mitochondrial unfolded protein response in epididymal white adipose tissue of mice. Free Radic Biol Med 108:704–714
Bohnert KR, Gallot YS, Sato S, Xiong G, Hindi SM, Kumar A (2016) Inhibition of ER stress and unfolding protein response pathways causes skeletal muscle wasting during cancer cachexia. FASEB J 30(9):3053–3068
Bohnert KR, McMillan JD, Kumar A (2018) Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and disease. J Cell Physiol 233(1):67–78
Bonaldo P, Sandri M (2013) Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 6(1):25–39
Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, Quiroga C, Rodriguez AE, Verdejo HE, Ferreira J, Iglewski M, Chiong M, Simmen T, Zorzano A, Hill JA, Rothermel BA, Szabadkai G, Lavandero S (2011) Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci 124(Pt 13):2143–2152
Chami M, Oules B, Szabadkai G, Tacine R, Rizzuto R, Paterlini-Brechot P (2008) Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol Cell 32(5):641–651
Cristea A, Qaisar R, Edlund PK, Lindblad J, Bengtsson E, Larsson L (2010) Effects of aging and gender on the spatial organization of nuclei in single human skeletal muscle cells. Aging Cell 9(5):685–697
Culver BH, Graham BL, Coates AL, Wanger J, Berry CE, Clarke PK, Hallstrand TS, Hankinson JL, Kaminsky DA, MacIntyre NR, McCormack MC, Rosenfeld M, Stanojevic S, Weiner DJ (2017) Recommendations for a standardized pulmonary function report. An official American thoracic society technical statement. Am J Respir Crit Care Med 196(11):1463–1472
Deldicque L, Cani PD, Philp A, Raymackers JM, Meakin PJ, Ashford ML, Delzenne NM, Francaux M, Baar K (2010) The unfolded protein response is activated in skeletal muscle by high-fat feeding: potential role in the downregulation of protein synthesis. Am J Physiol Endocrinol Metab 299(5):E695-705
Doucet M, Dube A, Joanisse DR, Debigare R, Michaud A, Pare ME, Vaillancourt R, Frechette E, Maltais F (2010) Atrophy and hypertrophy signalling of the quadriceps and diaphragm in COPD. Thorax 65(11):963–970
Ferrington DA, Krainev AG, Bigelow DJ (1998) Altered turnover of calcium regulatory proteins of the sarcoplasmic reticulum in aged skeletal muscle. J Biol Chem 273(10):5885–5891
Fu Y, Cai J, Xi M, He Y, Zhao Y, Zheng Y, Zhang Y, Xi J, He Y (2020) Neuroprotection effect of astragaloside IV from 2-DG-induced endoplasmic reticulum stress. Oxid Med Cell Longev 2020:9782062
Gea J, Agusti A, Roca J (2013) Pathophysiology of muscle dysfunction in COPD. J Appl Physiol 114(9):1222–1234
Gosselink R, Troosters T, Decramer M (2000) Distribution of muscle weakness in patients with stable chronic obstructive pulmonary disease. J Cardiopulm Rehabil 20(6):353–360
Green HJ, Burnett M, Duhamel TA, D’Arsigny C, O’Donnell DE, Webb KA, Ouyang J (2008) Abnormal sarcoplasmic reticulum Ca2+-sequestering properties in skeletal muscle in chronic obstructive pulmonary disease. Am J Physiol Cell Physiol 295(2):C350-357
Gurley JM, Ilkayeva O, Jackson RM, Griesel BA, White P, Matsuzaki S, Qaisar R, Van Remmen H, Humphries KM, Newgard CB, Olson AL (2016) Enhanced GLUT4-dependent glucose transport relieves nutrient stress in obese mice through changes in lipid and amino acid metabolism. Diabetes 65(12):3585–3597
Han X, Xu T, Fang Q, Zhang H, Yue L, Hu G, Sun L (2021) Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol 44:102010
Hayashi G, Labelle-Dumais C, Gould DB (2018) Use of sodium 4-phenylbutyrate to define therapeutic parameters for reducing intracerebral hemorrhage and myopathy in Col4a1 mutant mice. Dis Model Mech 11(7):1–7
Huertas A, Palange P (2011) COPD: a multifactorial systemic disease. Ther Adv Respir Dis 5(3):217–224
Kim HC, Mofarrahi M, Hussain SN (2008) Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 3(4):637–658
Lamboley CR, Murphy RM, McKenna MJ, Lamb GD (2013) Endogenous and maximal sarcoplasmic reticulum calcium content and calsequestrin expression in type I and type II human skeletal muscle fibres. J Physiol 591(23):6053–6068
Lassar AB (2017) Finding MyoD and lessons learned along the way. Semin Cell Dev Biol 72:3–9
Lee CS, Hanna AD, Wang H, Dagnino-Acosta A, Joshi AD, Knoblauch M, Xia Y, Georgiou DK, Xu J, Long C, Amano H, Reynolds C, Dong K, Martin JC, Lagor WR, Rodney GG, Sahin E, Sewry C, Hamilton SL (2017) A chemical chaperone improves muscle function in mice with a RyR1 mutation. Nat Commun 8:14659
Li H, Malhotra S, Kumar A (2008) Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med (Berl) 86(10):1113–1126
Lindner P, Christensen SB, Nissen P, Moller JV, Engedal N (2020) Cell death induced by the ER stressor thapsigargin involves death receptor 5, a non-autophagic function of MAP1LC3B, and distinct contributions from unfolded protein response components. Cell Commun Signal 18(1):12
Ma Y, Hendershot LM (2004) ER chaperone functions during normal and stress conditions. J Chem Neuroanat 28(1–2):51–65
Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L (2011) Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol 3(6):1–30
Mirza S, Clay RD, Koslow MA, Scanlon PD (2018) COPD Guidelines: a review of the 2018 GOLD report. Mayo Clin Proc 93(10):1488–1502
Nagaraju K, Casciola-Rosen L, Lundberg I, Rawat R, Cutting S, Thapliyal R, Chang J, Dwivedi S, Mitsak M, Chen YW, Plotz P, Rosen A, Hoffman E, Raben N (2005) Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum 52(6):1824–1835
Oldfors A, Moslemi AR, Jonasson L, Ohlsson M, Kollberg G, Lindberg C (2006) Mitochondrial abnormalities in inclusion-body myositis. Neurology 66(2 Suppl 1):S49-55
Peng Q, Liu Y, Kong X, Xian J, Ye L, Yang L, Guo S, Zhang Y, Zhou L, Xiang T (2021) The novel methylation biomarker SCARA5 sensitizes cancer cells to DNA damage chemotherapy drugs in NSCLC. Front Oncol 11:666589
Qaisar RKA, Muhammad T (2020) Circulating biomarkers of handgrip strength and lung function in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 15:311–315
Qaisar R, Renaud G, Morine K, Barton ER, Sweeney HL, Larsson L (2012) Is functional hypertrophy and specific force coupled with the addition of myonuclei at the single muscle fiber level? FASEB J 26(3):1077–1085
Qaisar R, Renaud G, Hedstrom Y, Pollanen E, Ronkainen P, Kaprio J, Alen M, Sipila S, Artemenko K, Bergquist J, Kovanen V, Larsson L (2013) Hormone replacement therapy improves contractile function and myonuclear organization of single muscle fibres from postmenopausal monozygotic female twin pairs. J Physiol 591(9):2333–2344
Qaisar R, Bhaskaran S, Premkumar P, Ranjit R, Natarajan KS, Ahn B, Riddle K, Claflin DR, Richardson A, Brooks SV, Van Remmen H (2018) Oxidative stress-induced dysregulation of excitation-contraction coupling contributes to muscle weakness. J Cachexia Sarcopenia Muscle 9(5):1003–1017
Qaisar R, Bhaskaran S, Ranjit R, Sataranatarajan K, Premkumar P, Huseman K, Van Remmen H (2019) Restoration of SERCA ATPase prevents oxidative stress-related muscle atrophy and weakness. Redox Biol 20:68–74
Qaisar R, Karim A, Elmoselhi AB (2020a) Muscle unloading: a comparison between spaceflight and ground-based models. Acta Physiol (Oxf) 228(3):e13431
Qaisar R, Karim A, Muhammad T, Shah I (2020b) Circulating biomarkers of accelerated sarcopenia in respiratory diseases. Biology (Basel) 9(10):1–14
Qaisar R, Karim A, Muhammad T, Shah I, Khan J (2021a) Prediction of sarcopenia using a battery of circulating biomarkers. Sci Rep 11(1):8632
Qaisar R, Qayum M, Muhammad T (2021b) Reduced sarcoplasmic reticulum Ca(2+) ATPase activity underlies skeletal muscle wasting in asthma. Life Sci 273:119296
Rayavarapu S, Coley W, Nagaraju K (2012) Endoplasmic reticulum stress in skeletal muscle homeostasis and disease. Curr Rheumatol Rep 14(3):238–243
Sataranatarajan K, Qaisar R, Davis C, Sakellariou GK, Vasilaki A, Zhang Y, Liu Y, Bhaskaran S, McArdle A, Jackson M, Brooks SV, Richardson A, Van Remmen H (2015) Neuron specific reduction in CuZnSOD is not sufficient to initiate a full sarcopenia phenotype. Redox Biol 5:140–148
Testelmans D, Crul T, Maes K, Agten A, Crombach M, Decramer M, Gayan-Ramirez G (2010) Atrophy and hypertrophy signalling in the diaphragm of patients with COPD. Eur Respir J 35(3):549–556
Torres S, Solsona-Vilarrasa E, Nunez S, Matias N, Insausti-Urkia N, Castro F, Casasempere M, Fabrias G, Casas J, Enrich C, Fernandez-Checa JC, Garcia-Ruiz C (2021) Acid ceramidase improves mitochondrial function and oxidative stress in Niemann-Pick type C disease by repressing STARD1 expression and mitochondrial cholesterol accumulation. Redox Biol 45:102052
Vogiatzis I, Simoes DC, Stratakos G, Kourepini E, Terzis G, Manta P, Athanasopoulos D, Roussos C, Wagner PD, Zakynthinos S (2010) Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur Respir J 36(2):301–310
World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194
Wu J, Ruas JL, Estall JL, Rasbach KA, Choi JH, Ye L, Bostrom P, Tyra HM, Crawford RW, Campbell KP, Rutkowski DT, Kaufman RJ, Spiegelman BM (2011) The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1alpha/ATF6alpha complex. Cell Metab 13(2):160–169
Wust RC, Degens H (2007) Factors contributing to muscle wasting and dysfunction in COPD patients. Int J Chron Obstruct Pulmon Dis 2(3):289–300
Yue-Chun L, Gu XH, Li-Sha G, Zhou DP, Xing C, Guo XL, Pan LL, Song SY, Yu LL, Chen GY, Lin JF, Chu MP (2021) Vagus nerve plays a pivotal role in CD4+ T cell differentiation during CVB3-induced murine acute myocarditis. Virulence 12(1):360–376
Zhang J, Li Y, Jiang S, Yu H, An W (2014) Enhanced endoplasmic reticulum SERCA activity by overexpression of hepatic stimulator substance gene prevents hepatic cells from ER stress-induced apoptosis. Am J Physiol Cell Physiol 306(3):C279-290
Zhou L, Zhang C, Yang X, Liu L, Hu J, Hou Y, Tao H, Sugimura H, Chen Z, Wang L, Chen K (2021) Melatonin inhibits lipid accumulation to repress prostate cancer progression by mediating the epigenetic modification of CES1. Clin Transl Med 11(6):e449
Zhu E, Wu H, Chen W, Qin Y, Liu J, Fan S, Ma S, Wu K, Mao Q, Luo C, Qin Y, Yi L, Ding H, Zhao M, Chen J (2021) Classical swine fever virus employs the PERK- and IRE1-dependent autophagy for viral replication in cultured cells. Virulence 12(1):130–149
Zito E (2019) Targeting ER stress/ER stress response in myopathies. Redox Biol 26:101232
Funding
This work was supported by Target (1901090168) and competitive grants (1901090157) from the University of Sharjah to Rizwan Qaisar.
Author information
Authors and Affiliations
Contributions
Conceptualization by RQ, patients’ recruitment and data collection by MQ and TM, data analysis by RQ, funding acquisition by RQ, and preparation of the manuscript by RQ. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All experimental protocols were approved by the hospital research ethics committees of GMC (REC-19-04-1801, dated: 04/03/2019). The study was conducted under the tenets of the Declaration of Helsinki (World Medical Association 2013).
Consent to participate
Written informed consent was obtained from all study participants.
Consent for publication
All authors agreed to the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qaisar, R., Ustrana, S., Muhammad, T. et al. Sarcopenia in pulmonary diseases is associated with elevated sarcoplasmic reticulum stress and myonuclear disorganization. Histochem Cell Biol 157, 93–105 (2022). https://doi.org/10.1007/s00418-021-02043-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-021-02043-3