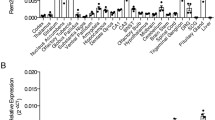
Abstract
Ras homolog enriched in brain (RHEB1) is a member within the superfamily of GTP-binding proteins encoded by the RAS oncogenes. RHEB1 is located at the crossroad of several important pathways including the insulin-signaling pathways and thus plays an important role in different physiological processes. To understand better the physiological relevance of RHEB1 protein, the expression pattern of RHEB1 was analyzed in both embryonic (at E3.5–E16.5) and adult (1-month old) mice. RHEB1 immunostaining and X-gal staining were used for wild-type and Rheb1 gene trap mutant mice, respectively. These independent methods revealed similar RHEB1 expression patterns during both embryonic and postnatal developments. Ubiquitous uniform RHEB1/β-gal and/or RHEB1 expression was seen in preimplantation embryos at E3.5 and postimplantation embryos up to E12.5. Between stages E13.5 and E16.5, RHEB1 expression levels became complex: In particular, strong expression was identified in neural tissues, including the neuroepithelial layer of the mesencephalon, telencephalon, and neural tube of CNS and dorsal root ganglia. In addition, strong expression was seen in certain peripheral tissues including heart, intestine, muscle, and urinary bladder. Postnatal mice have broad spatial RHEB1 expression in different regions of the cerebral cortex, subcortical regions (including hippocampus), olfactory bulb, medulla oblongata, and cerebellum (particularly in Purkinje cells). Significant RHEB1 expression was also viewed in internal organs including the heart, intestine, urinary bladder, and muscle. Moreover, adult animals have complex tissue- and organ-specific RHEB1 expression patterns with different intensities observed throughout postnatal development. Its expression level is in general comparable in CNS and other organs of mouse. Thus, the expression pattern of RHEB1 suggests that it likely plays a ubiquitous role in the development of the early embryo with more tissue-specific roles in later development.






Similar content being viewed by others
References
ACVP PMTDMD (2012) Comparative anatomy and histology. a mouse and human atlas, 1st edn. Academic Press as imprimt of Elsevier, Burlington
Aspuria PJ, Tamanoi F (2004) The Rheb family of GTP-binding proteins. Cell Signal 16(10):1105–1112. doi:10.1016/j.cellsig.2004.03.019
Baker MD, Ezzati M, Aloisio GM, Tarnawa ED, Cuevas I, Nakada Y, Castrillon DH (2014) The small GTPase Rheb is required for spermatogenesis but not oogenesis. Reproduction 147(5):615–625. doi:10.1530/REP-13-0304
Basso AD, Mirza A, Liu G, Long BJ, Bishop WR, Kirschmeier P (2005) The farnesyl transferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR signaling. Role in FTI enhancement of taxane and tamoxifen anti-tumor activity. J Biol Chem 280(35):31101–31108. doi:10.1074/jbc.M503763200
Buerger C, DeVries B, Stambolic V (2006) Localization of Rheb to the endomembrane is critical for its signaling function. Biochem Biophys Res Commun 344(3):869–880. doi:10.1016/j.bbrc.2006.03.220
Chakraborty S, Mohiyuddin SM, Gopinath KS, Kumar A (2008) Involvement of TSC genes and differential expression of other members of the mTOR signaling pathway in oral squamous cell carcinoma. BMC Cancer 8:163. doi:10.1186/1471-2407-8-163
Davis LI (1995) The nuclear pore complex. Annu Rev Biochem 64:865–896. doi:10.1146/annurev.bi.64.070195.004245
Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD (2011) The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol 12(4):295–303. doi:10.1038/ni.2005
Eom M, Han A, Yi SY, Shin JJ, Cui Y, Park KH (2008) RHEB expression in fibroadenomas of the breast. Pathol Int 58(4):226–232. doi:10.1111/j.1440-1827.2008.02215.x
Fedorov LM (2004) Discovery of genes active in embryogenesis by gene trapping. Ontogenez 35(4):307–313
Fedorov LM, Haegel-Kronenberger H, Hirchenhain J (1997) A comparison of the germline potential of differently aged ES cell lines and their transfected descendants. Transgenic Res 6(3):223–231
Fedorov LM, Tyrsin OY, Krenn V, Chernigovskaya EV, Rapp UR (2001) Tet-system for the regulation of gene expression during embryonic development. Transgenic Res 10(3):247–258
Friedel RH, Soriano P (2010) Gene trap mutagenesis in the mouse. Methods Enzymol 477:243–269. doi:10.1016/S0076-6879(10)77013-0
Friedrich G, Soriano P (1991) Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev 5(9):1513–1523
Goorden SM, Hoogeveen-Westerveld M, Cheng C, van Woerden GM, Mozaffari M, Post L, Duckers HJ, Nellist M, Elgersma Y (2011) Rheb is essential for murine development. Mol Cell Biol 31(8):1672–1678. doi:10.1128/MCB.00985-10
Gromov PS, Madsen P, Tomerup N, Celis JE (1995) A novel approach for expression cloning of small GTPases: identification, tissue distribution and chromosome mapping of the human homolog of rheb. FEBS Lett 377(2):221–226
Hamada S, Hara K, Hamada T, Yasuda H, Moriyama H, Nakayama R, Nagata M, Yokono K (2009) Upregulation of the mammalian target of rapamycin complex 1 pathway by Ras homolog enriched in brain in pancreatic beta-cells leads to increased beta-cell mass and prevention of hyperglycemia. Diabetes 58(6):1321–1332. doi:10.2337/db08-0519
Hanker AB, Mitin N, Wilder RS, Henske EP, Tamanoi F, Cox AD, Der CJ (2010) Differential requirement of CAAX-mediated posttranslational processing for Rheb localization and signaling. Oncogene 29(3):380–391. doi:10.1038/onc.2009.336
Heard JJ, Fong V, Bathaie SZ, Tamanoi F (2014) Recent progress in the study of the Rheb family GTPases. Cell Signal 26(9):1950–1957. doi:10.1016/j.cellsig.2014.05.011
Jiang H, Vogt PK (2008) Constitutively active Rheb induces oncogenic transformation. Oncogene 27(43):5729–5740. doi:10.1038/onc.2008.180
Kaufman MH (1995) The Atlas of mouse development, 2nd edn. Academic Press, Burlington
Kozak M (2007) Some thoughts about translational regulation: forward and backward glances. J Cell Biochem 102(2):280–290. doi:10.1002/jcb.21464
Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, Meyerson M, Gabriel SB, Lander ES, Getz G (2014) Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505(7484):495–501. doi:10.1038/nature12912
Li Y, Inoki K, Guan KL (2004) Biochemical and functional characterizations of small GTPase Rheb and TSC2 GAP activity. Mol Cell Biol 24(18):7965–7975. doi:10.1128/MCB.24.18.7965-7975.2004
Lin JR, Liu Z, Hu J (2014) Computational identification of post-translational modification-based nuclear import regulations by characterizing nuclear localization signal-import receptor interaction. Proteins 82(10):2783–2796. doi:10.1002/prot.24642
Lu ZH, Shvartsman MB, Lee AY, Shao JM, Murray MM, Kladney RD, Fan D, Krajewski S, Chiang GG, Mills GB, Arbeit JM (2010) Mammalian target of rapamycin activator RHEB is frequently overexpressed in human carcinomas and is critical and sufficient for skin epithelial carcinogenesis. Cancer Res 70(8):3287–3298. doi:10.1158/0008-5472.CAN-09-3467
Ma D, Bai X, Guo S, Jiang Y (2008) The switch I region of Rheb is critical for its interaction with FKBP38. J Biol Chem 283(38):25963–25970. doi:10.1074/jbc.M802356200
Magdaleno S, Jensen P, Brumwell CL, Seal A, Lehman K, Asbury A, Cheung T, Cornelius T, Batten DM, Eden C, Norland SM, Rice DS, Dosooye N, Shakya S, Mehta P, Curran T (2006) BGEM: an in situ hybridization database of gene expression in the embryonic and adult mouse nervous system. PLoS Biol 4(4):e86. doi:10.1371/journal.pbio.0040086
Manning BD, Cantley LC (2003) Rheb fills a GAP between TSC and TOR. Trends Biochem Sci 28(11):573–576. doi:10.1016/j.tibs.2003.09.003
Mavrakis KJ, Zhu H, Silva RL, Mills JR, Teruya-Feldstein J, Lowe SW, Tam W, Pelletier J, Wendel HG (2008) Tumorigenic activity and therapeutic inhibition of Rheb GTPase. Genes Dev 22(16):2178–2188. doi:10.1101/gad.1690808
Mizuguchi M, Takashima S (2001) Neuropathology of tuberous sclerosis. Brain Dev 23(7):508–515
Mizuki N, Kimura M, Ohno S, Miyata S, Sato M, Ando H, Ishihara M, Goto K, Watanabe S, Yamazaki M, Ono A, Taguchi S, Okumura K, Nogami M, Taguchi T, Ando A, Inoko H (1996) Isolation of cDNA and genomic clones of a human Ras-related GTP-binding protein gene and its chromosomal localization to the long arm of chromosome 7, 7q36. Genomics 34(1):114–118
Nardella C, Chen Z, Salmena L, Carracedo A, Alimonti A, Egia A, Carver B, Gerald W, Cordon-Cardo C, Pandolfi PP (2008) Aberrant Rheb-mediated mTORC1 activation and Pten haploinsufficiency are cooperative oncogenic events. Genes Dev 22(16):2172–2177. doi:10.1101/gad.1699608
Patel PH, Thapar N, Guo L, Martinez M, Maris J, Gau CL, Lengyel JA, Tamanoi F (2003) Drosophila Rheb GTPase is required for cell cycle progression and cell growth. J Cell Sci 116(Pt 17):3601–3610. doi:10.1242/jcs.00661
Paxinos KBJFG (2008) The mouse brain, 2008th edn. Academic Press, Burlington
Rosner M, Freilinger A, Hengstschlager M (2007) Akt regulates nuclear/cytoplasmic localization of tuberin. Oncogene 26(4):521–531. doi:10.1038/sj.onc.1209812
Saito K, Araki Y, Kontani K, Nishina H, Katada T (2005) Novel role of the small GTPase Rheb: its implication in endocytic pathway independent of the activation of mammalian target of rapamycin. J Biochem 137(3):423–430. doi:10.1093/jb/mvi046
Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320(5882):1496–1501. doi:10.1126/science.1157535
Schmick M, Kraemer A, Bastiaens PI (2015) Ras moves to stay in place. Trends Cell Biol 25(4):190–197. doi:10.1016/j.tcb.2015.02.004
Skarnes WC, Auerbach BA, Joyner AL (1992) A gene trap approach in mouse embryonic stem cells: the lacZ reported is activated by splicing, reflects endogenous gene expression, and is mutagenic in mice. Genes Dev 6(6):903–918
Sorokin AV, Kim ER, Ovchinnikov LP (2007) Nucleocytoplasmic transport of proteins. Biochemistry (Mosc) 72(13):1439–1457
Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, Breuer S, Thomas G, Hafen E (2003) Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol 5(6):559–565. doi:10.1038/ncb995
Sunabori T, Tokunaga A, Nagai T, Sawamoto K, Okabe M, Miyawaki A, Matsuzaki Y, Miyata T, Okano H (2008) Cell-cycle-specific nestin expression coordinates with morphological changes in embryonic cortical neural progenitors. J Cell Sci 121(Pt 8):1204–1212. doi:10.1242/jcs.025064
Tamai T, Yamaguchi O, Hikoso S, Takeda T, Taneike M, Oka T, Oyabu J, Murakawa T, Nakayama H, Uno Y, Horie K, Nishida K, Sonenberg N, Shah AM, Takeda J, Komuro I, Otsu K (2013) Rheb (Ras homologue enriched in brain)-dependent mammalian target of rapamycin complex 1 (mTORC1) activation becomes indispensable for cardiac hypertrophic growth after early postnatal period. J Biol Chem 288(14):10176–10187. doi:10.1074/jbc.M112.423640
Tee AR, Anjum R, Blenis J (2003a) Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase/Akt-dependent and -independent phosphorylation of tuberin. J Biol Chem 278(39):37288–37296. doi:10.1074/jbc.M303257200
Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J (2003b) Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol 13(15):1259–1268
Tian Q, Hanlon Newell AE, Wang Y, Olson SB, Fedorov LM (2011) Complex cytogenetic analysis of early lethality mouse embryos. Chromosome Res 19(4):567–574. doi:10.1007/s10577-011-9209-4
Uhlmann EJ, Li W, Scheidenhelm DK, Gau CL, Tamanoi F, Gutmann DH (2004) Loss of tuberous sclerosis complex 1 (Tsc1) expression results in increased Rheb/S6K pathway signaling important for astrocyte cell size regulation. Glia 47(2):180–188. doi:10.1002/glia.20036
Urano J, Tabancay AP, Yang W, Tamanoi F (2000) The Saccharomyces cerevisiae Rheb G-protein is involved in regulating canavanine resistance and arginine uptake. J Biol Chem 275(15):11198–11206
Wilson V, Manson L, Skarnes WC, Beddington RS (1995) The T gene is necessary for normal mesodermal morphogenetic cell movements during gastrulation. Development 121(3):877–886
Yadav RB, Burgos P, Parker AW, Iadevaia V, Proud CG, Allen RA, O’Connell JP, Jeshtadi A, Stubbs CD, Botchway SW (2013) mTOR direct interactions with Rheb-GTPase and raptor: sub-cellular localization using fluorescence lifetime imaging. BMC Cell Biol 14:3. doi:10.1186/1471-2121-14-3
Yamagata K, Sanders LK, Kaufmann WE, Yee W, Barnes CA, Nathans D, Worley PF (1994) rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J Biol Chem 269(23):16333–16339
Zheng H, Liu A, Liu B, Li M, Yu H, Luo X (2010) Ras homologue enriched in brain is a critical target of farnesyltransferase inhibitors in non-small cell lung cancer cells. Cancer Lett 297(1):117–125. doi:10.1016/j.canlet.2010.05.004
Zou J, Zhou L, Du XX, Ji Y, Xu J, Tian J, Jiang W, Zou Y, Yu S, Gan L, Luo M, Yang Q, Cui Y, Yang W, Xia X, Chen M, Zhao X, Shen Y, Chen PY, Worley PF, Xiao B (2011) Rheb1 is required for mTORC1 and myelination in postnatal brain development. Dev Cell 20(1):97–108. doi:10.1016/j.devcel.2010.11.020
Acknowledgments
We gratefully acknowledge Galina Fedorova, Sigrun Kirste, and Carolyn McCain for expert technical assistance, and Chris Corless and Louise Lanoue for excellent technical advice. We acknowledge the DNA core facility at OHSU for sequencing analysis and histopathology core at OHSU for the technical expertise. The work was partly supported by Medical Research Foundation (Portland, Oregon), Grant # 9003823, the Office of the Vice President for Research, at OHSU, Paul K. and Evelyn Richter Memorial Fund; Oregon and Southwest Washington, grant to J. L. S. and NBL-3 grant from Bundesministerium für Bildung und Forschung, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tian, Q., Smart, J.L., Clement, J.H. et al. RHEB1 expression in embryonic and postnatal mouse. Histochem Cell Biol 145, 561–572 (2016). https://doi.org/10.1007/s00418-015-1394-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-015-1394-3