Abstract
Background/aims
To evaluate the efficacy, safety and durability of intravitreal faricimab in patients with neovascular age-related macular degeneration (nAMD) with unsatisfactory response to traditional anti-vascular endothelial growth factor (anti-VEGF) agents.
Methods
Single-centre, prospective cohort study of all consecutive patients with nAMD who were switched to intravitreal faricimab from intravitreal ranibizumab or aflibercept, due to unsatisfactory treatment response (maximal fluid-free interval ≤ 8 weeks). Intravitreal faricimab was administered with a loading dose of four 4-weekly injections, followed by an 8-week extension. A treat and extend (T&E) regime was adopted thereafter. Primary outcome was the difference between the maximal fluid-free interval achieved with faricimab, and the one achieved before the switch. Morpho-functional outcomes were also assessed. Secondary outcome was accordance with clinical management when applying faricimab pivotal trial criteria versus our real-world T&E protocol, measured as a proportion.
Results
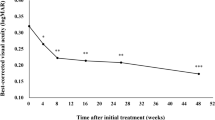
Twenty-six eyes of 26 patients with a median age of 82 years (range 77–85) were included. Patients were followed for 30.2 weeks (range 26.3–33.1). Maximal fluid-free interval after switch to faricimab (Mdn = 6.0 weeks; IQR = 4–8) was longer than the maximum interval before the switch (Mdn = 4.0 weeks; IQR = 4–4), p < 0.001. Comparing real-world T&E protocol with pivotal trial criteria, 8 (30.8%) eyes received the same clinical management while 18 (69.2%) eyes were kept at a shorter interval when following our T&E protocol. No serious adverse events were recorded.
Conclusions
Faricimab appears to increase the fluid-free interval and allow extension of dosing interval in patients with nAMD poorly responsive to traditional anti-VEGF drugs.





Similar content being viewed by others
References
Li JQ, Welchowski T, Schmid M et al (2020) Prevalence and incidence of age-related macular degeneration in Europe: a systematic review and meta-analysis. Br J Ophthalmol 104(8):1077–1084
Tan CS, Ngo WK, Chay IW et al (2022) Neovascular age-related macular degeneration (nAMD): a review of emerging treatment options. Clin Ophthalmol 16:917–933
Swiss Agency for Therapeutic Products (2022) Vabysmo Public Summary SwissPAR. https://www.swissmedic.ch/swissmedic/en/home/about-us/publications/public-summary-swiss-par/public-summary-swiss-par-vabysmo.html. Accessed 10 Aug 2023
Heier JS, Khanani AM, Quezada Ruiz C et al (2022) Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet Lond Engl 399(10326):729–740
Khanani AM, Aziz AA, Khan H et al (2023) The real-world efficacy and safety of faricimab in neovascular age-related macular degeneration: the TRUCKEE study–6 month results. Eye 37(17):3574–3581
Leung EH, Oh DJ, Alderson SE et al (2023) Initial real-world experience with faricimab in treatment-resistant neovascular age-related macular degeneration. Clin Ophthalmol Auckl NZ 17:1287–1293
Matsumoto H, Hoshino J, Nakamura K et al (2023) Short-term outcomes of intravitreal faricimab for treatment-naïve neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 261(10):2945–2952
Stanga PE, Valentín-Bravo FJ, Stanga SEF et al (2023) Faricimab in neovascular AMD: first report of real-world outcomes in an independent retina clinic. Eye 37(15):3282–3289
Rush RB, Rush SW (2022) Intravitreal faricimab for aflibercept-resistant neovascular age-related macular degeneration. Clin Ophthalmol Auckl NZ 16:4041–4046
Cheng AM, Joshi S, Banoub RB et al (2023) Faricimab effectively resolves intraretinal fluid and preserves vision in refractory, recalcitrant, and nonresponsive neovascular age-related macular degeneration. Cureus 15(6):e40100
Khanani AM, Guymer RH, Basu K et al (2021) TENAYA and LUCERNE: rationale and design for the phase 3 clinical trials of faricimab for neovascular age-related macular degeneration. Ophthalmol Sci 1(4):100076
Augsburger M, Sarra GM, Imesch P (2019) Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol 257(9):1889–1895
Li E, Donati S, Lindsley KB et al (2020) Treatment regimens for administration of anti‐vascular endothelial growth factor agents for neovascular age‐related macular degeneration. Cochrane Database Syst Rev 5(5):CD012208
Marquis LM, Mantel I (2020) Beneficial switch from aflibercept to ranibizumab for the treatment of refractory neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 258(8):1591–1596
Gale RP, Pearce I, Eter N et al (2020) Anatomical and functional outcomes following switching from aflibercept to ranibizumab in neovascular age-related macular degeneration in Europe: SAFARI study. Br J Ophthalmol 104(4):493–499
Holz FG, Schmitz-Valckenberg S, Wolf A et al (2022) A randomized, open-label, multicenter study of switching to brolucizumab with or without a loading dose for patients with suboptimal anatomically controlled neovascular age-related macular degeneration—the FALCON study. Graefes Arch Clin Exp Ophthalmol 260(8):2695–2702
Maloca P, Hasler PW, Barthelmes D et al (2018) Safety and feasibility of a novel sparse optical coherence tomography device for patient-delivered retina home monitoring. Transl Vis Sci Technol 7(4):8
Guymer RH, Markey CM, McAllister IL et al (2019) Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology 126(5):723–734
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the local ethics committee of Canton Ticino (2023–00653 CE 4340) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
G. Grimaldi provided consulting and/or speaker services for Apellis, Bayer and Roche. M. Menghini provided consulting and/or speaker services for Bayer, Roche, Novartis, Apellis and AbbVie, and is CMO and holds equity of Endogena Inc. All authors received an unrestricted research grant from Bayer AG Switzerland.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grimaldi, G., Cancian, G., Rizzato, A. et al. Intravitreal faricimab for neovascular age-related macular degeneration previously treated with traditional anti-VEGF compounds: a real-world prospective study. Graefes Arch Clin Exp Ophthalmol 262, 1151–1159 (2024). https://doi.org/10.1007/s00417-023-06319-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06319-3