Abstract
Purpose
The aim of this study was to evaluate the expression of placental growth factor (PLGF), neuropilin-1 (NP-1), and neuropilin-2 (NP-2) molecules in primary pterygium tissue compared with normal conjunctival tissue.
Methods
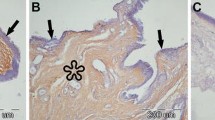
The records of 42 patients who underwent excision surgery with autografts for primary pterygium (pterygium group) and 20 patients who underwent conjunctival nevus excision surgery (control group) in the same period were reviewed retrospectively. The samples obtained from the pterygium tissues in the pterygium group and the clean conjunctival tissues adjacent to the nevus in the control group were collected from the archive. Immunohistochemical stains of the primary antibodies—1/100 diluted PLGF, NP-1, and NP-2 (Abcam Cambridge Science Park, UK)—were applied to all groups. Staining intensities and the percentage of positive cells in epithelial, endothelial, stromal, and inflammatory cells were analyzed by an experienced pathologist.
Results
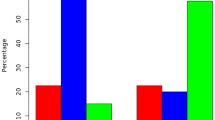
The positivity rates of PLGF and NP-2 expression in epithelial, endothelial, stromal, and inflammatory cells were found to be higher in the pterygium group than in the control group (PLGF: p < 0.001, p < 0.001, p = 0.001, and p < 0.001, respectively; NP-2: p < 0.001 for all). Staining intensities for PLGF and NP-2 were higher in the pterygium group than in the control group (PLGF: p < 0.001, p < 0.001, p = 0.005, and p < 0.001, respectively; NP-2: p < 0.001, p < 0.001, p = 0.001, and p < 0.001, respectively). However, no significant differences were found in any cell type in terms of NP-1 expression positivity rates (p = 0.730, p = 0.121, p = 0.524, and p = 0.624, respectively) or staining intensity (p = 0.716, p = 0.147, p = 0.147, and p = 0.780, respectively).
Conclusion
PLGF and NP-2 levels were found to be higher in pterygium tissue, while there was no difference in NP-1. These results indicate the possible roles of NP-2 and PLGF in primary pterygium.




Similar content being viewed by others
References
Di Girolamo N, Chui J, Coroneo MT, Wakefield D (2004) Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res 23(2):195–228. https://doi.org/10.1016/j.preteyeres.2004.02.002
Chui J, Di Girolamo N, Wakefield D, Coroneo MT (2008) The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul Surf 6(1):24–43. https://doi.org/10.1016/s1542-0124(12)70103-9
Lee DH, Cho HJ, Kim JT, Choi JS, Joo CK (2001) Expression of vascular endothelial growth factor and inducible nitric oxide synthase in pterygia. Cornea 20(7):738–742. https://doi.org/10.1097/00003226-200110000-00013
Aspiotis M, Tsanou E, Gorezis S, Ioachim E, Skyrlas A, Stefaniotou M et al (2007) Angiogenesis in pterygium: study of microvessel density, vascular endothelial growth factor, and thrombospondin-1. Eye (Lond) 21(8):1095–1101. https://doi.org/10.1038/sj.eye.6702495
Liang K, Jiang Z, Zhao B, Shen J, Huang D, Tao L (2012) The expression of vascular endothelial growth factor in mast cells promotes the neovascularisation of human pterygia. Br J Ophthalmol 96(9):1246–1251. https://doi.org/10.1136/bjophthalmol-2012-301540
Hoyama E, Viveiros MM, Shiratori C, de Oliveira DE, Padovani CR, Selva D et al (2015) Expression of vascular endothelial growth factor (VEGF) in macrophages, fibroblasts, and endothelial cells in pterygium treated with 5-Fluorouracil. Semin Ophthalmol 30(3):171–176. https://doi.org/10.3109/08820538.2013.835838
Ziche M, Maglione D, Ribatti D, Morbidelli L, Lago CT, Battisti M et al (1997) Placenta growth factor-1 is chemotactic, mitogenic, and angiogenic. Lab Investig 76(4):517–531
Bagri A, Tessier-Lavigne M, Watts RJ (2009) Neuropilins in tumor biology. Clin Cancer Res 15(6):1860–1864. https://doi.org/10.1158/1078-0432.CCR-08-0563
Herzog Y, Kalcheim C, Kahane N, Reshef R, Neufeld G (2001) Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech Dev 109(1):115–119. https://doi.org/10.1016/s0925-4773(01)00518-4
Ling S, Liang L, Lin H, Li W, Xu J (2012) Increasing lymphatic microvessel density in primary pterygia. Arch Ophthalmol 130(6):735–742. https://doi.org/10.1001/archophthalmol.2012.293
Fukuhara J, Kase S, Ohashi T, Ando R, Dong Z, Noda K et al (2013) Expression of vascular endothelial growth factor C in human pterygium. Histochem Cell Biol 139(2):381–389. https://doi.org/10.1007/s00418-012-1019-z
Caunt M, Mak J, Liang WC, Stawicki S, Pan Q, Tong RK et al (2008) Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell 13(4):331–342. https://doi.org/10.1016/j.ccr.2008.01.029
Seifert P, Sekundo W (1998) Capillaries in the epithelium of pterygium. Br J Ophthalmol 82(1):77–81. https://doi.org/10.1136/bjo.82.1.77
Lutty GA, McLeod DS, Merges C, Diggs A, Plouét J (1996) Localization of vascular endothelial growth factor in human retina and choroid. Arch Ophthalmol 114(8):971–977. https://doi.org/10.1001/archopht.1996.01100140179011
Marcovich AL, Morad Y, Sandbank J, Huszar M, Rosner M, Pollack A et al (2002) Angiogenesis in pterygium: morphometric and immunohistochemical study. Curr Eye Res 25(1):17–22. https://doi.org/10.1076/ceyr.25.1.17.9959
Jin J, Guan M, Sima J, Gao G, Zhang M, Liu Z et al (2003) Decreased pigment epithelium-derived factor and increased vascular endothelial growth factor levels in pterygia. Cornea 22(5):473–477. https://doi.org/10.1097/00003226-200307000-00015
Detorakis ET, Zaravinos A, Spandidos DA (2010) Growth factor expression in ophthalmic pterygia and normal conjunctiva. Int J Mol Med 25(4):513–516. https://doi.org/10.3892/ijmm_00000371
Di Girolamo N, Kumar RK, Coroneo MT, Wakefield D (2002) UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci 43(11):3430–3437
Chiang CC, Cheng YW, Lin CL, Lee H, Tsai FJ, Tseng SH et al (2007) Cyclooxygenase 2 expression in pterygium. Mol Vis 13:635–638
Awdeh RM, DeStafeno JJ, Blackmon DM, Cummings TJ, Kim T (2008) The presence of T-lymphocyte subpopulations (CD4 and CD8) in pterygia: evaluation of the inflammatory response. Adv Ther 25(5):479–487. https://doi.org/10.1007/s12325-008-0056-4
Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D et al (2003) Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med 9(7):936–943. https://doi.org/10.1038/nm884
Snuderl M, Batista A, Kirkpatrick ND, Ruiz de Almodovar C, Riedemann L, Walsh EC et al (2013) Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell 152(5):1065–1076. https://doi.org/10.1016/j.cell.2013.01.036
Perelman N, Selvaraj SK, Batra S, Luck LR, Erdreich-Epstein A, Coates TD et al (2003) Placenta growth factor activates monocytes and correlates with sickle cell disease severity. Blood 102(4):1506–1514. https://doi.org/10.1182/blood-2002-11-3422
Paavonen K, Puolakkainen P, Jussila L, Jahkola T, Alitalo K (2000) Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol 156(5):1499–1504. https://doi.org/10.1016/S0002-9440(10)65021-3
Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P et al (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 7(2):192–198. https://doi.org/10.1038/84643
Lin H, Luo L, Ling S, Chen W, Liu Z, Zhong X et al (2013) Lymphatic microvessel density as a predictive marker for the recurrence time of pterygium: a three-year follow-up study. Mol Vis 19:166–173
Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P et al (2006) Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood 108(4):1243–1250. https://doi.org/10.1182/blood-2005-11-4447
Yuan L, Moyon D, Pardanaud L, Bréant C, Karkkainen MJ, Alitalo K, Eichmann A (2002) Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 129(20):4797–4806. https://doi.org/10.1242/dev.129.20.4797
Tang XL, Sun JF, Wang XY, Du LL, Liu P (2010) Blocking neuropilin-2 enhances corneal allograft survival by selectively inhibiting lymphangiogenesis on vascularized beds. Mol Vis 16:2354–2361
Tang X, Sun J, Du L, Du H, Wang L, Mai J, Zhang F, Liu P (2016) Neuropilin-2 contributes to LPS-induced corneal inflammatory lymphangiogenesis. Exp Eye Res 143:110–119. https://doi.org/10.1016/j.exer.2015.10.017
Funding
This study was supported by Necmettin Erbakan University Scientific Research Projects Coordination Unit (Project number: 211518003).
The work has been done without receiving any financial support from any third party.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. AOG, RO, and PO contributed to the acquisition of data. AOG and SB contributed to the analysis and interpretation of data. AOG and SB drafted the manuscript. RO and PO were involved in critical revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Clinical Research Ethics Committee of Necmettin Erbakan University and followed the tenets of the Declaration of Helsinki. (Decision No: 2020/1492).
Conflict interests
The authors declare that there is no conflict of interest.
The authors have no proprietary or financial interest in any product mentioned in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gundogan, A.O., Oltulu, R., Belviranli, S. et al. Expression of placental growth factor, neuropilin-1, and neuropilin-2 in primary pterygium tissue. Graefes Arch Clin Exp Ophthalmol 262, 957–965 (2024). https://doi.org/10.1007/s00417-023-06280-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06280-1