Abstract
Purpose
Rheumatoid arthritis (RA) is the most common inflammatory joint disease, and hydroxychloroquine (HCQ) is an established treatment. The extent to which HCQ impacts ocular microvascular vessel density (VD) in patients with RA without evidence of HCQ retinopathy has not yet been conclusively clarified. The main aim of this study was to evaluate VD measured by optical coherence tomography angiography (OCTA) in patients with RA treated with HCQ.
Methods
The VD data of the 3 × 3 mm OCT angiogram (RTVue XR Avanti, Optovue Inc., Fremont, California, USA) as well as the retinal thickness (RT) data of patients with RA (n = 30) and healthy controls (n = 30) were extracted and analyzed. The study group was further divided into patients undergoing HCQ treatment for > 5 years (high-risk-group) and < 5 years (low-risk group).
Results
Patients with RA showed no evidence of VD reduction compared to the control group in all obtained regions (p > 0.05). Correlation analysis revealed no dependency between VD, RT, and HCQ therapy duration or cumulative HCQ dose (p > 0.05). High-risk patients showed a decreased VD in the superficial quadrant of the superficial capillary plexus compared to low-risk-patients (p = 0.022). Whole-en-face RT was reduced in the high-risk group compared to the control group (p = 0.019).
Conclusion
Our study showed no evidence that HCQ diminishes VD in patients with RA without HCQ retinopathy measured by OCTA. However, RA patients with a long duration of therapy showed a significantly reduced RT. Our results suggest that quantitative VD analysis by OCTA may not be suitable for early detection of HCQ retinopathy and that the focus on detecting early HCQ retinopathy should be on intensive and sequential OCT diagnostics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by persistent synovitis, systemic inflammation, and autoantibodies production, such as rheumatoid factor and anticitrullinated peptides, and can affect a variety of tissues [1]. Although RA primarily affects the joints, it is known to frequently affect extra-articular structures including, but not limited to, the heart, vascular structures, and kidneys [1, 2]. RA is conventionally treated with a group of drugs known as disease-modifying anti-rheumatic drugs (DMARDs), which include glucocortidocids, methotrexate, leflunomide, sulfasalazine, and hydroxychloroquine (HCQ) [2]. HCQ is a well-known therapy able to prevent autophagosome–lysosome fusion, which is a critical step in the pathomechanism of rheumatic diseases [3]. This property allows considering HCQ as one of the major drugs used in autoimmune rheumatic conditions, especially in patients with systemic lupus erythematodes [4]. However, in patients with RA, antimalarials including HCQ are mainly reserved for patients with mild RA, as no new evidence regarding a good efficacy of hydroxychloroquine was found for RA in general and the historic studies had shown only weak clinical and no structural efficacy [5]. Nonetheless, there is still a large proportion of patients with RA being treated with HCQ. Although HCQ is generally well-tolerated, it might cause irreversible visual loss due to retinal toxicity. The risk of toxicity at recommended doses up to 5 years of treatment is < 1%, but it increases sharply after approximately 5 years of therapy and an overall prevalence of 7.5% was identified in patients taking HCQ for greater than 5 years, rising to almost 20% after 20 years of treatment [6, 7]. Early detection of HCQ retinopathy is crucial, since its damage to the retinal pigment epithelium (RPE) is irreversible and can progress even after cessation of the therapy [7]. The revised recommendations on screening for chloroquine and HCQ retinopathy of the American Academy of Ophthalmology from 2016 include automated visual fields plus spectral-domain optical coherence tomography (SD-OCT) as primary screening tests for detection of early HCQ retinopathy. Additional useful objective screening tests are multifocal electroretinogram (mfERG) providing objective corroboration for visual fields as well as fundus autofluorescence (FAF) showing topographical damage. [7]
In 2020, the Royal College of Ophthalmologists (RCOphth) also published revised recommendations on monitoring for chloroquine and HCQ stating that Humphrey visual field testing is no longer recommended as a first-line monitoring test, but that monitoring for HCQ retinopathy should involve SD-OCT imaging and widefield FAF imaging for all patients, if available [8]. These revised recommendations in recent years show that early detection of HCQ retinopathy remains challenging and new developments in multimodal imaging should be investigated for potential suitability as an early detection tool.
Optical coherence tomography angiography (OCTA) provides a three-dimensional visualization of choroidal and retinal microvascular layers as well as the possibility of quantification of the vessel density (VD) of macular capillary plexuses in a non-invasive way [9].
Different study groups demonstrated VD alterations in patients under HCQ treatment including patients with RA compared to healthy controls using OCTA [10,11,12,13]. These studies, however, show different, partly contradictory results with either reduced or increased VD and with the alterations occurring in different capillary plexus and regions. Also, they either included patients with other connective tissue diseases like systemic lupus erythematodes (SLE), did not analyze choriocapillary VD, or did not perform a correlation analysis between VD data and cumulative HCQ dose or duration of HCQ therapy [10,11,12,13].
Thus, the aim of the present OCTA study was to evaluate retinal and choriocapillary VD differences in patients with RA undergoing HCQ therapy in comparison to healthy controls and to correlate the data with the cumulative HCQ dose and duration of HCQ therapy.
Methods
30 eyes of 30 patients with RA and 30 eyes of 30 gender and age matched healthy controls were consecutively included in this study. The healthy controls were recruited from subjects who visited our outpatient clinic for routine examinations and had no ophthalmological or systemic diseases. The study was approved by the Ethics Committee of the University of Muenster, North Rhine Westphalia, Germany. Before performing any examination, the study protocol was explained in detail and all participants signed an informed consent form. The study adhered to the tenets of the Declaration of Helsinki. Patients and controls with media opacities preventing high-quality imaging, vitreoretinal disease, previous retinal surgery, macular edema, glaucoma, or neurological disease were excluded from the study. All study participants underwent an ophthalmic examination including anterior segment examination, binocular fundus examination, and OCTA imaging. In the patient group, 10–2 visual field testing (standard automated perimetry), SD-OCT using the Spectralis OCT (Heidelberg Engineering, Germany), FAF, and multifocal electroretinogram were performed to rule out HCQ associated retinopathy. Only patients with no signs of HCQ toxicity were included in this study. Patients treated with HCQ for > 5 years were classified as the “high-risk group” (n = 21); patients with a HCQ treatment for < 5 years were classified as the “low-risk group” (n = 9). This classification was based on the revised recommendations on screening for HCQ therapy of the American Academy of Ophthalmology on screening for chloroquine and HCQ retinopathy which define a duration of use > 5 years as a factor of increasing risk of HCQ-retinopathy [7]. Other major risk factors like renal disease, tamoxifen use, excessive daily dose by weight, and retinal and macular diseases were defined as exclusion criteria. For the correlation analysis of the VD, RT, and the cumulative dose of HCQ, cumulative dose data were extracted from all patient records (n = 30).
Optical coherence tomography angiography
OCTA imaging was performed using the RTVue XR Avanti system with AngioVue (Optovue Inc, Fremont, California, USA). Split-spectrum amplitude-decorrelation angiography (SSADA) was used to extract the OCTA information. OCTA imaging technology has been described in detail elsewhere [9]. The macula was imaged using a 3 × 3 mm scan. The automated segmentation was checked by an expert examiner before data analysis. All examinations were performed under the same conditions at the same location by an expert examiner. Images with poor signal strength (signal strength index (SSI) < 6) were not included in the study. In case of sufficient image quality of both eyes, the selection of the eye was randomised. Otherwise, the eye with the better SSI was selected. After imaging, the VD in SCP and DCP OCT angiogram, the RT, and the FAZ were analyzed using the integrated device software (AngioAnalytics, version 2017.1.0.151, Optovue Inc, Fremont, California, USA). To determine VD values of the choriocapillaris, OCTA images were exported. Using Adobe Photoshop CS6 (Adobe Systems, Inc., California), images were converted into grey scales. Each pixel was attributed to a value that represents the strength of the decorrelation signal. VD in the choriocapillaris was calculated as the mean decorrelation value of all pixels in the images (arbitrary unit, AU) [14].
Statistics
IBM SPSS® Statistics 28 for Windows (IBM Corporation, Somers, NY, USA) was used for statistical analyses. The data was tested for normality distribution using the Shapiro–Wilk test, and all data did fit a normal distribution. The data are therefore presented as mean value ± standard deviation. The control and study group were compared using the two-sided independent sample t-test for normally distributed variables, and the degree of correlation between two variables was expressed as the Pearson’s correlation coefficient (r). For evaluating differences between the three subgroups (low-risk group, high-risk group, and control group), a closed testing procedure was used. Specifically, a one-way analysis of variance (ANOVA) was performed followed by a post hoc analysis via two-sided independent sample t-tests. Inferential statistics are intended to be exploratory (hypothesis-generating), not confirmatory, and are interpreted accordingly. The comparison-wise type-I error rate is controlled instead of the experiment-wise error rate. The chosen level of significance was p < 0.05.
Results
There was no significant difference in age between RA patients and healthy controls (p = 0.203) and between patients of the high-risk group and the low-risk group (p = 0.192). The demographic characteristics of patients and healthy controls are shown in Table 1. For the comparisons of HCQ therapy duration and cumulative doses between the low-risk group and the high-risk group the values of Hedges g were 1.271 and 1.007, which puts our data into the moderate to large effect sizes. It is about the equivalent of ROC AUCs of 0.815 and 0.762 [15].
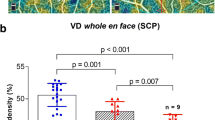
VD analysis showed no statistically significant differences of the VD data between the study group and control group (p > 0.05) (Fig. 1; Table 2). Correlation analysis between the HCQ therapy duration, cumulative HCQ dose, and VD or retinal thickness data revealed no statistically significant dependency, either (p > 0.05) (Supplemental Table 1).
A–F Optical coherence tomography angiography (OCTA) and retinal thickness (RT) map. Exemplary 3 × 3 mm OCTA images of the macula (A, B: whole-en-face image of the superficial capillary plexus; C, D color-coded images indicating the analyzed regions) and RT map (E, F) of a patient with rheumatoid arthritis (top row) and an age-matched healthy control (bottom row)
The whole-en-face (WEF) retinal thickness, however, was significantly reduced in the study group compared to healthy controls (RTWEF study group: 306.30 µm ± 13.74 µm, RTWEF control group: 314.43 µm ± 11.63 µm, p = 0.016; Table 2). An analysis of the three groups low-risk, high-risk, and control group showed that the WEF-RT was only significantly reduced in the high-risk-group (RTWEF high-risk-group: 305.62 µm ± 14.19 µm, RTWEF control group: 314.43 µm ± 11.63 µm, p = 0.019; RTWEF low-risk-group: 307.89 ± 13.32 µm, RTWEF control group: 314.43 µm ± 11.63 µm, p = 0.160).
A comparison of the VD data between the low-risk-group and the high-risk-group revealed a significantly reduced VD in the superior quadrant of the SCP in the high-risk-group (VDSCP sup low-risk group: 50.55% ± 1.52%, VDSCP sup high-risk-group: 47.44% ± 5.35%, p = 0.022) and a non-significant trend in the parafoveal area of the SCP (VDSCP parafovea low risk group: 47.02% ± 4.36%, VDSCP parafovea high-risk-group: 49.63% ± 2.16%, p = 0.1) (Table 3).
Discussion
In this quantitative OCTA study, our results suggest no VD differences between RA patients undergoing HCQ therapy and healthy controls. Particularly, we could not show a correlation between the VD and cumulative HCQ dose or HCQ therapy duration. Previous OCTA studies in the literature show different results and conclusions on the influence of HCQ on VD [11,12,13]. Hence, the impact of HCQ on VD as well as the relevance of OCTA diagnostics in these patients is not yet fully clarified. Details of the patient characteristics and the main results of the aforementioned studies are summarized in Table 4.
Our results are in line with those of Remoli Sargues et al., who demonstrated even an increase of VD in patients with various autoimmune diseases who underwent HCQ treatment and therefore suggest that HCQ retinal toxicity is not vascular mediated [13]. However, they only included 17 patients with RA and only 2 of them belonged to the high-risk group. In contrast to that, Ozek et al. included 40 patients with RA and showed a reduced VD in the deep temporal and deep hemi-inferior vascular plexus in the high-risk group, suggesting a relevance of OCTA measurements to the early findings of HCQ toxicity [11].
In our analysis, there was no difference between the VD data of the control group and high-risk-group neither in the superficial nor in the deep vascular plexus. However, VD values in the deep retinal layer show a weaker repeatability compared to the VD values of the superficial retinal layer [16] and OCTA imaging in the deep vascular layer is more challenging and affected by artefacts [17, 18].
Other studies showing a reduction of the VD using OCTA in patients under HCQ treatment included patients with various diseases and did not differentiate between patients with RA and other autoimmune diseases, such as SLE which is known to be associated with immune complex-mediated microangiopathy and might cause retinopathy itself [13]. We believe it is very important to create homogenous study populations in these kind of quantitative OCTA studies, since it cannot be concluded from these findings whether the observed alterations were genuinely caused by the underlying disease or if these changes could be influenced or biased by the HCQ therapy. Moreover, as suggested in other OCTA studies on patients with SLE, HCQ might have a protective role on preserving microvasculature in autoimmune diseases while reducing the disease activity [13, 19].
To the best of our knowledge, none of the mentioned studies analyzing solely RA patients performed a correlation analysis between VD data and cumulative HCQ dose or HCQ therapy duration (Table 4). Although one might assume that a high cumulative dose or long therapy duration has an impact on VD, we did not find a tendency in our correlation analysis. Since none of our patients showed signs of HCQ retinopathy, we postulate that neither the cumulative HCQ dose nor the HCQ therapy duration do have an impact on VD in these patients and might not be suitable for determination of early retinopathy in these patients.
When comparing VD data of low-risk and high-risk patients, VD was significantly reduced in the superior quadrant of the high-risk group. We believe this result should be taken with caution and not equated with very early alterations/thinning of retinal structures by OCT analysis which suggest the inferior quadrant to be affected earlier than the superior quadrant [20, 21].
In patients with long HCQ therapy duration, we demonstrated a reduced whole-en-face RT compared to the control group. Latest publications of Kim et al. and Marmor et al. emphasize the sensitivity of RT analysis in detecting HCQ retinopathy [22,23,24]. Marmor et al. even suggest that sequential RT analysis might be the earliest diagnostic sign of HCQ retinopathy [24]. In our study, population without clinical evidence for HCQ retinopathy, only the whole-en-face RT showed a significant difference between the high-risk-group and the healthy control group [23]. Due to the recent findings of the publications mentioned above, this could lead to the conclusion that this might be a very early sign for clinically not yet detected retinopathies [22,23,24].
Our study has some limitations worth noting: First, it is a cross-sectional study. Therefore, we cannot comment on the value of VD measurements for evaluation of disease progression. Further studies on OCTA imaging in RA patients undergoing HCQ treatment with a longitudinal design should be performed in future. A second limitation is the small sample size of the low-risk group. It might be that some of the negative findings (i.e. lack of significant differences) were due to the limited sample size. However, correcting for small cohort size with Hedges g still revealed moderate to large effect sizes. Moreover, compared to other OCTA studies evaluating VD in RA patients, our study has one of the largest numbers of RA patients with a long duration of therapy and high cumulative doses. In addition, the patients in the current study accounted a rather homogeneous age- and gender-matched study population.
In conclusion, the results of our study show no sign of reduction of microvascular density in patients with RA regardless of the duration of HCQ therapy. Moreover, VD data did not correlate with the duration of therapy or cumulative dose. Since the existing literature on VD alterations in patients undergoing HCQ treatment show very controversial results, we suggest that VD analysis using OCTA seems not to be a suitable additional diagnostic mean in detecting early signs of HCQ retinopathy in patients with RA. Considering the latest study results on RT diminution in patients with HCQ therapy in the literature and our presented results, the main focus on detecting early HCQ retinopathy should remain on intensive and sequential OCT diagnostics.
Data availability
All data of this study are available from the corresponding author upon reasonable request.
References
Celia AI, Colafrancesco S, Barbati C et al (2022) Autophagy in rheumatic diseases: role in the pathogenesis and therapeutic approaches. Cells 11(8):1359. https://doi.org/10.3390/cells11081359
Nazir AM, Koganti B, Gupta K et al (2021) Evaluating the use of hydroxychloroquine in treating patients with rheumatoid arthritis. Cureus 13(11):e19308. https://doi.org/10.7759/cureus.19308
Nirk EL, Reggiori F, Mauthe M (2020) Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol Med 12(8):e12476. https://doi.org/10.15252/emmm.202012476
Fanouriakis A, Kostopoulou M, Alunno A et al (2019) 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 78(6):736–745. https://doi.org/10.1136/annrheumdis-2019-215089
Smolen JS, Landewé RBM, Bijlsma JWJ et al (2020) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 79(6):685–699. https://doi.org/10.1136/annrheumdis-2019-216655
Yusuf IH, Sharma S, Luqmani R, Downes SM (2017) Hydroxychloroquine retinopathy. Eye (Lond) 31(6):828–845. https://doi.org/10.1038/eye.2016.298
Marmor MF, Kellner U, Lai TYY et al (2016) Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 Revision). Ophthalmology 123:1386–1394. https://doi.org/10.1016/j.ophtha.2016.01.058
Yusuf IH, Foot B, Lotery AJ (2021) The Royal College of Ophthalmologists recommendations on monitoring for hydroxychloroquine and chloroquine users in the United Kingdom (2020 revision): executive summary. Eye (Lond) 35(6):1532–1537. https://doi.org/10.1038/s41433-020-01380-2
Spaide RF, Fujimoto JG, Waheed NK (2015) Image artifacts in optical coherence angiography. Retina 35(11):2163–2180. https://doi.org/10.1097/IAE.0000000000000765
Goker YS, Atılgan CU, Tekin K et al (2019) The validity of optical coherence tomography angiography as a screening test for the early detection of retinal changes in patients with hydroxychloroquine therapy. Curr Eye Res 44(3):311–315. https://doi.org/10.1080/02713683.2018.1545912
Ozek D, Onen M, Karaca EE et al (2019) The optical coherence tomography angiography findings of rheumatoid arthritis patients taking hydroxychloroquine. Eur J Ophthalmol 29(5):532–537. https://doi.org/10.1177/1120672118801125
Iacono P, Da Pozzo S, Bedendo A et al (2022) OCT retinal angiography features in patients with rheumatoid arthritis: a pilot study. Eur J Ophthalmol 32(4):2433–2439. https://doi.org/10.1177/11206721211035626
Remolí Sargues L, Monferrer Adsuara C, Castro Navarro V et al (2022) New insights in pathogenic mechanism of hydroxychloroquine retinal toxicity through optical coherence tomography angiography analysis. Eur J Ophthalmol 11206721221076312. https://doi.org/10.1177/11206721221076313
Alten F, Heiduschka P, Clemens CR et al (2016) Exploring choriocapillaris under reticular pseudodrusen using OCT-Angiography. Graefes Arch Clin Exp Ophthalmol 254(11):2165–2173. https://doi.org/10.1007/s00417-016-3375-1
Rice ME, Harris GT (2005) Comparing effect sizes in follow-up studies: ROC Area, Cohen’s d, and r. Law Hum Behav 29(5):615–620. https://doi.org/10.1007/s10979-005-6832-7
Mihailovic N, Brand C, Lahme L et al (2018) Repeatability, reproducibility and agreement of foveal avascular zone measurements using three different optical coherence tomography angiography devices. PLoS One 13(10):e0206045. https://doi.org/10.1371/journal.pone.0206045
Spaide RF, Curcio CA (2017) Evaluation of segmentation of the superficial and deep vascular layers of the retina by optical coherence tomography angiography instruments in normal eyes. JAMA Ophthalmol 135(3):259–262. https://doi.org/10.1001/jamaophthalmol.2016.5327
Fenner BJ, Tan GSW, Tan ACS et al (2018) Identification of imaging features that determine quality and repeatability of retinal capillary plexus density measurements in OCT angiography. Br J Ophthalmol 102(4):509–514. https://doi.org/10.1136/bjophthalmol-2017-310700
Mihailovic N, Leclaire MD, Eter N, Brücher VC (2020) Altered microvascular density in patients with systemic lupus erythematosus treated with hydroxychloroquine-an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol 258(10):2263–2269. https://doi.org/10.1007/s00417-020-04788-4
Marmor MF, Melles RB (2014) Disparity between visual fields and optical coherence tomography in hydroxychloroquine retinopathy. Ophthalmology 121(6):1257–1262. https://doi.org/10.1016/j.ophtha.2013.12.002
Ulviye Y, Betul T, Nur TH, Selda C (2013) Spectral domain optical coherence tomography for early detection of retinal alterations in patients using hydroxychloroquine. Indian J Ophthalmol 61(4):168–171. https://doi.org/10.4103/0301-4738.112161
Kim KE, Ahn SJ, Woo SJ et al (2021) Use of OCT retinal thickness deviation map for hydroxychloroquine retinopathy screening. Ophthalmology 128(1):110–119. https://doi.org/10.1016/j.ophtha.2020.06.021
Marmor MF, Durbin M, de Sisternes L, Pham BH (2021) Sequential retinal thickness analysis shows hydroxychloroquine damage before other screening techniques. Retin Cases Brief Rep 15(3):185–196. https://doi.org/10.1097/ICB.0000000000001108
Melles RB, Marmor MF (2022) Rapid macular thinning is an early indicator of hydroxychloroquine retinal toxicity. Ophthalmology 129(9):1004–1013. https://doi.org/10.1016/j.ophtha.2022.05.002
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the University of Muenster, North Rhine Westphalia, Germany, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esser, E.L., Zimmermann, J.A., Storp, J.J. et al. Retinal microvascular density analysis in patients with rheumatoid arthritis treated with hydroxychloroquine. Graefes Arch Clin Exp Ophthalmol 261, 1433–1442 (2023). https://doi.org/10.1007/s00417-022-05946-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05946-6