Abstract
Purpose
This study aims to evaluate the efficacy and safety of the PreserFlo MicroShunt (Santen, Osaka, Japan) in lowering intraocular pressure (IOP) in childhood glaucoma patients with previous failed glaucoma surgeries.
Methods
This is a prospective case review of consecutive PreserFlo procedures performed in childhood glaucoma patients after failed surgeries. Age, sex, diagnosis, and previous glaucoma surgeries, as well as visual acuity, IOP, and treatment in the preoperative visit and all follow-up visits were collected. Outcome measures included IOP reduction from baseline, mean IOP change from baseline at month 6, medication use at 6 months, complications, adverse events, and need for further procedures.
Results
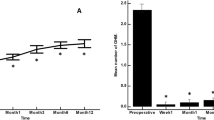
Fourteen patients were included, 8 (57%) males and 6 (43%) females; the mean age was 27.5 ± 13.5 years. Nine patients (64%) had at least two trabeculectomies, and 6 patients (43%) had at least one trabeculectomy and a glaucoma drainage implant. The mean IOP change from baseline was 11.3 ± 4.9 mmHg at 12 months. At 12 months, 12 patients (86%) presented ≥ 20% IOP lowering from baseline, and 11 patients (79%) presented ≥ 30%. The mean medication count decreased from 3.9 ± 0.7 (baseline) to 0.7 ± 1.3 (12 months). No intraoperative complications were reported. No adverse events were noted. No secondary filtration surgery was required, although bleb needling was required in one case, 1 month after the surgery.
Conclusions
PreserFlo with MMC can be used successfully to treat uncontrolled IOP in childhood glaucoma cases with previous failed surgeries. Larger studies with longer follow-up are needed to further explore the role of the device in resistant childhood glaucoma cases.



Similar content being viewed by others
References
Thau A, Lloyd M, Freedman S et al (2018) New classification system for pediatric glaucoma: implications for clinical care and a research registry. Curr Opin Ophthalmol 29:385–394. https://doi.org/10.1097/ICU.0000000000000516
Papadopoulos M, Vanner EA, Grajewski AL et al (2020) Ophthalmol Glaucoma 3:145–157. https://doi.org/10.1016/j.ogla.2019.12.007
Mocan MC, Mehta AA, Aref AA (2019) Update in genetics and surgical management of primary congenital glaucoma. Turkish J Ophthalmol 49:347–355. https://doi.org/10.4274/tjo.galenos.2019.28828
Chen TC, Chen PP, Francis BA et al (2014) Pediatric glaucoma surgery: a report by the American Academy of Ophthalmology. Ophthalmology 121:2107–2115. https://doi.org/10.1016/j.ophtha.2014.05.010
Papadopoulos M, Edmunds B, Fenerty C, Khaw PT (2014) Childhood glaucoma surgery in the 21st century. Eye (Lond) 28:931–943. https://doi.org/10.1038/eye.2014.140
Gillmann K, Mansouri K (2020) Minimally invasive glaucoma surgery: where is the evidence? Asia-Pacific J Ophthalmol 9:203–214. https://doi.org/10.1097/APO.0000000000000294
Batlle JF, Fantes F, Riss I et al (2016) Three-year follow-up of a novel aqueous humor microshunt. J Glaucoma 25:e58–e65. https://doi.org/10.1097/IJG.0000000000000368
Sadruddin O, Pinchuk L, Angeles R, Palmberg P (2019) Ab externo implantation of the MicroShunt, a poly (styrene-block-isobutylene-block-styrene) surgical device for the treatment of primary open-angle glaucoma: a review. Eye Vis (Lond) 6:36. https://doi.org/10.1186/s40662-019-0162-1
Tanimoto SA, Brandt JD (2006) Options in pediatric glaucoma after angle surgery has failed. Curr Opin Ophthalmol 17:132–137. https://doi.org/10.1097/01.icu.0000193091.60185.27
Fulcher T, Chan J, Lanigan B et al (1996) Long-term follow up of primary trabeculectomy for infantile glaucoma. Br J Ophthalmol 80:499–502. https://doi.org/10.1136/bjo.80.6.499
Mandal AK, Prasad K, Naduvilath TJ (1999) Surgical results and complications of mitomycin C-augmented trabeculectomy in refractory developmental glaucoma. Ophthalmic Surg Lasers 30:473–480
Budenz DL, Gedde SJ, Brandt JD et al (2004) Baerveldt glaucoma implant in the management of refractory childhood glaucomas. Ophthalmology 111:2204–2210. https://doi.org/10.1016/j.ophtha.2004.05.017
Baker ND, Barnebey HS, Moster MR et al (2021) Ab-externo MicroShunt versus trabeculectomy in primary open-angle glaucoma: one-year results from a 2-year randomized, multicenter study. Ophthalmology 128:1710–1721. https://doi.org/10.1016/j.ophtha.2021.05.023
Batlle JF, Corona A, Albuquerque R (2020) Long-term results of the PRESERFLO® MicroShunt in patients with primary open-angle glaucoma from a single-center non-randomized study. J Glaucoma 3:4–5. https://doi.org/10.1097/IJG.0000000000001734
Beckers HJM, Aptel F, Webers CAB et al (2021) Safety and effectiveness of the PRESERFLO® MicroShunt in primary open-angle glaucoma: results from a 2-year multicenter study. Ophthalmol Glaucoma 5:195–209. https://doi.org/10.1016/j.ogla.2021.07.008
Durr GM, Schlenker MB, Samet S, Ahmed IIK (2020) One-year outcomes of stand-alone ab externo SIBS microshunt implantation in refractory glaucoma. Br J Ophthalmol 1–9. https://doi.org/10.1136/bjophthalmol-2020-317299
Quaranta L, Micheletti E, Carassa R et al (2021) Efficacy and safety of PreserFlo® MicroShunt after a failed trabeculectomy in eyes with primary open-angle glaucoma: a retrospective study. Adv Ther 38:4403–4412. https://doi.org/10.1007/s12325-021-01811-w
Schlenker MB, Durr GM, Michaelov E, Ahmed IIK (2020) Intermediate outcomes of a novel standalone ab externo SIBS Microshunt with mitomycin C. Am J Ophthalmol 215:141–153. https://doi.org/10.1016/j.ajo.2020.02.020
Baker ND, Stiles M, Vold S et al (2020) Safety and effectiveness of MicroShunt implantation versus trabeculectomy: 1-year results from a randomized, multicenter study. Paper presented at the American Glaucoma Society (AGS) Annual Meeting February 27–March 1, 2020, Washington, DC, USA
Scheres LMJ, Kujovic-Aleksov S, Ramdas WD, et al., (2020) XEN® Gel Stent compared to PRESERFLOTM MicroShunt implantation for primary open-angle glaucoma: two-year results. Acta Ophthalmol 5. https://doi.org/10.1111/aos.14602
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Hospital Clinico San Carlos, with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and approved by the Hospital Clinico San Carlos Committee.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
B Burgos-Blasco, C Gines-Gallego, L Perucho-Gonzalez, Federico Saenz-Frances, L Morales-Fernandez, and CD Mendez-Hernandez have participated as coinvestigators in studies regarding the PreserFlo implant promoted by Santen.
J Garcia-Feijoo has received research funding from Santen, Allergan, Glaukos, Alcon, AJL, Thea, Sight Science, Heidelberg, J&J, B&L, Pfizer, Zeiss, Ivantis, and iStar and is Advisory Board of Santen, Alcon, Allergan, Alimera, Thea, Glaukos, and iStar.
JM Martinez de la Casa has received research funding from Santen, Ivantis, Allergan, Pfizer, Glaukos, and Thea; is advisory boards of Allergan, Santen, Alcon, Pfizer, Novartis, Thea, B&L, Glaukos, AJL, and Visufarma; and is a Speaker for Allergan, Santen, Alcon, Pfizer, Novartis, Thea, B&L, Glaukos, and ICare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Burgos-Blasco, B., García-Feijóo, J., Gines-Gallego, C. et al. Efficacy and safety of the PreserFlo implant with mitomycin C in childhood glaucoma after previous failed glaucoma surgeries. Graefes Arch Clin Exp Ophthalmol 261, 1349–1357 (2023). https://doi.org/10.1007/s00417-022-05939-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05939-5