Abstract
Purpose
This study reported 11 cases of new-onset acute uveitis following coronavirus disease 2019 (COVID-19) vaccination.
Methods
This retrospective observational case study included 11 eyes of 11 patients with acute uveitis after the COVID-19 vaccination. We only included patients with new-onset uveitis. The medical records of the patients from January 2021 to January 2022 were reviewed.
Results
The mean age of the participants was 51.81 years, and all patients demonstrated anterior chamber reaction with keratic precipitates in the affected eye. The mean duration between vaccination and uveitis was 8.27 days. Seven patients developed uveitis after receiving the second dose of vaccination, and four developed uveitis after receiving the third dose of vaccination. Five patients showed posterior synechiae, and three patients showed hypopyon. After treatment with topical 1% prednisolone acetate eye drops and systemic prednisolone, inflammation was adequately controlled and quickly resolved.
Conclusions
COVID-19 vaccination with messenger RNA and viral vector vaccines may cause acute anterior uveitis. Although initially severe, uveitis responded well to steroid therapy with no visual impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Coronavirus disease 2019 (COVID-19) has caused serious social and economic losses globally [1]. COVID-19 vaccines were rapidly developed based on previous vaccines for diseases such as the Middle East respiratory syndrome and severe acute respiratory syndrome [2]. Several vaccines were introduced during the pandemic, including messenger RNA (mRNA) vaccines such as mRNA-1273 (Moderna, Inc., Cambridge, MA) and BNT162b1 (Pfizer Inc., New York, BioNTech, Inc., Mainz, Germany) [3], as well as viral vector vaccines such as ChAdOX1 (Oxford-AstraZeneca), Ad26.COV2.S (Johnson and Johnson, New Brunswick, NJ), and BBIBP-CorV (Sinopharm, Beijing, China) [4,5,6]. These vaccines prevented asymptomatic and symptomatic infections, decreased disease severity, and reduced morbidity as well as mortality rates [7,8,9].
Nevertheless, systemic side effects, such as headache, fatigue, injection-site pain, and thrombosis, have been reported [10, 11]. Regarding COVID-19 vaccine-induced ocular complications, few studies on graft rejection after endothelial corneal transplant [12, 13] and uveitis [14, 15] have been reported to date. These studies reported a possible causal relationship between the vaccination and the occurrence of uveitis; however, there has been no strong evidence to prove it. Moreover, the number of reported cases is insufficient to be statistically significant, necessitating the requirement of more detailed reports.
In this retrospective case series, we report the clinical manifestations, treatment, and prognosis of 11 eyes of 11 patients with acute-onset uveitis after COVID-19 vaccination with no previous history of uveitis or any autoimmune diseases that can cause uveitis.
Methods
Study population
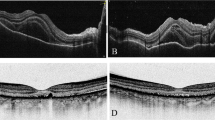
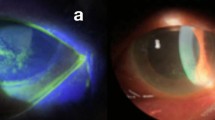
This case-series study was conducted at the Sanggye Paik Hospital of Inje University of Korea, Seoul, Korea. The study protocol was approved by the Inje University Institutional Review Board (IRB no. SGP-202202010). This study was conducted in accordance with the Declaration of Helsinki. The inclusion criteria were as follows: (1) patients who received any of the currently introduced COVID-19 vaccines (ChAdOX1, mRNA-1273, and Ad26.COV2.S); (2) patients who developed any signs of active anterior uveitis (keratic precipitates, anterior chamber cells, vitreous cells, and hypopyon) within a month of receiving the vaccination. The exclusion criteria were as follows: (1) any past history of uveitis; (2) any ocular surgery within 90 days before the diagnosis of uveitis; (3) any abnormal laboratory findings suggestive of uveitis-related systemic disease, except for the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level; (4) involvement of the posterior segment on fundus photographs, such as Vogt-Koyanagi-Harada syndrome (VKH), white dot syndrome, and retinal vasculitis (Fig. 1).
Diagnosis of uveitis
The patients were diagnosed with uveitis based on an ocular examination, including slit-lamp examination, fundus examination, non-contact tonometry, optical coherence tomography, best-corrected visual acuity (BCVA), and systemic laboratory examination. Laboratory tests for complete blood cell count, electrolytes, urinary analysis, routine blood examination, ESR, CRP level, serology, rheumatoid factor, CMV immunoglobulin IgG and IgM, human leukocyte antigen-B27 (HLA-B27), antistreptolysin O (ASO), antinuclear antibody (ANA), angiotensin-converting enzyme (ACE), anti-neutrophil cytoplasmic antibody (ANCA), toxoplasmosis IgG and IgM, and toxocariasis IgG. Chest radiography was also performed.
Treatment
All patients were administered 1% prednisolone acetate every 2 h and topical 1% atropine sulfate (Isopto® atropine, Alcon) twice a day. Oral prednisolone was administered in patients with 4 + anterior chamber inflammation, hypopyon, and/or 2 + vitritis. The initial dose of systemic prednisolone was 1 mg/kg/day and was tapered thereafter based on the inflammation. Response to therapy was defined as a decrease in anterior chamber inflammation and vitritis.
Results
Patient demographics
The baseline clinical characteristics and treatment outcomes of the study participants are shown in Table 1. All 11 participants had unilateral manifestations. The mean age was 51.81 ± 14.9 years, ranging from 21 to 72 years. The mean logMAR BCVA was 0.78 ± 0.42, and the mean intraocular pressure (IOP) was 14.81 ± 2.99 mmHg by non-contact tonometry. Fundus examination of one patient (patient 10) showed an epiretinal membrane. Five patients had vitritis, and hypopyon was observed in three patients. Five patients had posterior synechiae at the initial visit.
COVID-19 vaccines
All patients received at least a second dose of the COVID-19 vaccination, and four patients received a third dose. The mean period between vaccination and the onset of uveitis was 8.27 ± 4.47 days, ranging from 2 to 14 days. Five patients developed uveitis after receiving the second dose of BNT162b1, one patient developed uveitis after receiving the second dose of ChAdOX1, and uveitis occurred in one patient after receiving the second dose of Ad26.COV2.S. Three patients had onset of uveitis following the administration of the third dose of BNT162b1, and uveitis occurred in one patient after the administration of the second dose of ChAdOX1 and the third dose of BNT162b1 (Table 1).
Patient treatment outcomes
All participants received 1% prednisolone acetate every 2 h and 1% atropine sulfate twice daily. Six patients received systemic prednisolone. The mean final logMAR BCVA was 0.14 ± 0.29, and the mean IOP was 15.90 ± 3.17 mmHg by non-contact tonometry. Anterior chamber inflammation and vitritis resolved in all patients. The mean treatment period was 4.63 ± 1.43 weeks (Table 1).
Discussion
In this single-center case series, we analyzed 11 eyes of 11 patients with acute-onset uveitis following COVID-19 vaccination. We included patients who experienced the first episode of uveitis but had no abnormal findings on laboratory tests. Uveitis in patients with a previous history of uveitis or abnormalities on laboratory examinations for uveitis is less likely to be caused by vaccination. Therefore, to analyze the effect of vaccination on uveitis, it is necessary to reduce the selection bias and minimize the effects of other variables.
The association between vaccinations and various types of ocular inflammation has been reported. Various vaccines are associated with anterior and intermediate uveitis, Vogt-Koyanagi-Harada disease, and multiple evanescent white dot syndrome [16,17,18,19,20]. Currently, four different COVID-19 vaccines have been developed. In this study, two types of COVID-19 vaccines were analyzed: mRNA vaccine and virus vector vaccines ChAdOX1 and Ad26.COV2.S. The inactivated COVID-19 vaccine BBIBP-CorV (Sinopharm, Beijing, China) and protein subunit vaccine NVXCoV2373 (Novavax) were excluded from this study as they were not introduced in South Korea during the study period. In this study, eight patients developed uveitis after receiving the BNT162b2 mRNA SARS-CoV-2 vaccine, and one patient each developed uveitis after receiving the ChAdOX1 and Ad26.COV2.S virus vector vaccines. Uveitis occurred in one patient after receiving the second dose of ChAdOX1 and the third dose of BNT162b1. There was no difference in anterior chamber reaction, hypopyon, and vitritis between the mRNA SARS-CoV-2 vaccine and virus vector vaccine groups. Few studies on uveitis following the administration of BNT162b2 mRNA vaccination have been reported [15, 21, 22]. Recently, Rabinovitch et al. reported cases of uveitis after BNT162b2 mRNA SARS-CoV-2 vaccination [15]. The exact pathogenesis of uveitis following vaccination remains unclear. Cunningham proposed a mechanism based on the molecular similarities between uveal self-peptides and vaccine peptides, delayed-type hypersensitivity, and immune responses against vaccine adjuvants [23]. Steinemann et al. suggested that increased vascular permeability after vaccination affected the immunologic capability of the cornea. Immune complex deposition in the uvea and iris initiates a local inflammatory response [24]. Innate immunity is stimulated by adjuvants in mRNA vaccines through endosolic or cytoplasmic nucleic acid receptors [25]. Nucleic acid metabolism and processing may be altered in various autoimmune diseases. In return, immunization may induce an immune response [26, 27]. Such a mechanism may be considered one reason for COVID-19 vaccines triggering uveitis. In our study, all participants showed a favorable response to prednisolone, indicating an association between uveitis and vaccine-induced immune response.
In this study, all patients developed uveitis after receiving at least a second dose of the COVID-19 vaccine, 63.63% developed uveitis after receiving the second dose of vaccination, and 36.36% developed uveitis after receiving the third dose of vaccination. None of the participants developed acute uveitis after the first dose. These results are slightly different than those from the previous study by Rabinovitch et al., which reported that 32 and 68% of patients developed uveitis after receiving the first and second doses of BNT162b2 [15]. This difference may be due to the presence of some participants with a history of uveitis or uveitis-related systemic disease. Among the eight patients with uveitis following the administration of the first dose of the vaccine in Rabinovitch’s report, four patients had a previous history of uveitis, and one patient had a disease associated with uveitis. Such patients are possibly more susceptible to developing uveitis after the vaccination. Moreover, patients with a past history of uveitis are more likely to recognize uveitis-related symptoms and seek treatment. It should also be highlighted that the higher degree of reactogenicity related to the second or the third dose may explain the lower rate of occurrence of uveitis after the first dose. Polack et al. reported that systemic side effects occurred more frequently after the second dose of vaccination than after the first dose of vaccination because of the higher degree of reactogenicity connected with the second dose [27]. The development of acute uveitis after the second vaccination dose may be due to the same reason. In our study, all uveitis cases occurred shortly (range, 2–14 days) after vaccination. Vaccination may have contributed to the occurrence of uveitis based on the temporal association between vaccination and uveitis onset.
Polack et al. demonstrated that systemic side effects were more frequent in younger (< 55 years) participants [27]. In our study, the mean age was 51.81 ± 14.9 years (range, 21–72 years). Patient 2, a 21-year-old female patient, showed the shortest duration for the development of uveitis and the most severe anterior chamber inflammation with hypopyon and vitritis. The second youngest patient (patient 5, 32-year-old) also showed four signs of positive anterior chamber inflammation and vitritis. Younger patients tended to have more severe anterior chamber inflammation and vitritis. Although initially severe, all patients responded well to topical and systemic steroid therapy without vision-threatening complications. In patient 10, the final logMAR BCVA was 1.0 because of a preexisting epiretinal membrane.
The limitations of this study are as follows: the small sample size makes statistical analysis difficult, and the absence of a control group. Moreover, the causal relationship between the vaccination and the occurrence of uveitis is very difficult to prove at this moment, although it is very likely. The WHO described the causality assessment of suspected drug adverse as certain, probable, and possible [28]. Clinical response to the withdrawal of the medicine must be demonstrated for the adverse drug reaction to be classified as certain. However, vaccination has a long-lasting effect on the immune system, and the effect cannot be withdrawn. Therefore, defining the relationship between the vaccination and the occurrence of uveitis as certain causality would be very difficult. In contrast, probable causality is defined as a clinical event occurring after a reasonable duration following drug administration that is unlikely to be due to any concurrent disease or other drugs. Following this definition, all participants in our manuscript had probable causalities as they had temporal relationships between the events and were proven to have no previous history of uveitis or uveitis-related systemic disease.
In conclusion, mRNA and virus vector COVID-19 vaccines may provoke acute uveitis. In patients complaining of congestion or impaired vision after vaccination, the anterior chamber should be evaluated for inflammation to check for uveitis, particularly after the administration of the second vaccination dose.
References
Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ (2021) What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis 21:e26–e35
Aslam S, Goldstein DR, Vos R, Gelman AE, Kittleson MM, Wolfe C, Danziger-Isakov L (2021) COVID-19 vaccination in our transplant recipients: the time is now. J Heart Lung Transplant 40:169–171
Calina DAO, Docea D, Petrakis D, Egorov AM, Ishmukhametov AA, GabibovShtilmanKostoffCarvalhoVincetiSpandidosTsatsakis AGMIRFMDAA (2020) Towards effective COVID19 vaccines: updates, perspectives and challenges (review). Int J Mol Med 46:3–16
Ramasamy MN, Minassian AM, Ewer KJ et al (2021) Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 396:1979–1993
Voysey M, Costa Clemens SA, Madhi SA et al (2021) Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 397:881–891
Wang H, Zhang Y, Huang B et al (2020) Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell 182:713–721
Cook TM (2021) Roberts JV (2021) Impact of vaccination by priority group on UK deaths, hospital admissions and intensive care admissions from COVID-19. Anaesthesia 76:608–616
Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, Southern J, Swerdlow DL, Jodar L, Levy Y, Alroy-Preis S (2021) Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 15(397):1819–1829
Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O’Doherty M, Brown K, Cameron C, Stockton D, McMenamin J, Ramsay M (2021) Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 13(373):n1088
Kadali RAK, Janagama R, Peruru S, Malayala SV (2021) Side effects of BNT162b2 mRNA COVID-19 vaccine: a randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis 106:376–381
Chang JC, Hawley HB (2021) Vaccine-associated thrombocytopenia and thrombosis: venous endotheliopathy leading to venous combined micro-macrothrombosis. Med (Kaunas) 57:1163
Phylactou M, Li JO, Larkin DFP (2021) Characteristics of endothelial corneal transplant rejection following immunization with sars-cov-2 messenger RNA vaccine. Br J Ophthalmol 105:893–896
Abousy M, Bohm K, Prescott C, Bonsack JM, Rowhani-Farid A, Eghrari AO (2021) Bilateral ek rejection after COVID-19 vaccine. Eye Contact Lens 47:625–628
Pan L, Zhang Y, Cui Y, Wu X (2021) Bilateral uveitis after inoculation with COVID-19 vaccine: a case report. Int J Infect Dis 113:116–118
Rabinovitch T, Beb-Arie-Weintrob Y, Hareuveni-Blum T, Shaer B, Vishnevskia-Dai V, Shulman S, Newman H, Biadsy M, Masarwas D, Fischer N, Yovel O, Goldfeather-Ben Zaken S, Habot-Wilner Z (2021) Uveitis after the BNT162b2 mRNA vaccination against SARS-CoV-2 infection: a possible association. Retina 41:2462–2713
Sood AB, O’Keefe G, Bui D, Jain N (2019) Vogt-Koyanagi-Harada disease associated with hepatitis B vaccination. Ocul Immunol Inflamm 27:524–527
Abou-Samra A, Tarabishy AB (2019) Multiple evanescent white dot syndrome following intradermal influenza vaccination. Ocul Immunol Inflamm 27:528–530
Ng CC, Jumper JM, Cunningham ET Jr (2020) Multiple evanescent white dot syndrome following influenza immunization - a multimodal imaging study. Am J Ophthalmol Case Rep 19:100845
Marinho PM, Nascimento H, Romano A, Muccioli C, Belfort R Jr (2019) Diffuse uveitis and chorioretinal changes after yellow fever vaccination: a re-emerging epidemic. Int J Retina Vitreous 5:30
Biancardi AL, Moraes HV Jr (2019) Anterior and intermediate uveitis following yellow fever vaccination with fractional dose: case reports. Ocul Immunol Inflamm 27:521–523. 21. 21. Mudie LI, Zick JD, Dacey MS, Palestine AG (2021) Panuveitis following vaccination for COVID-19. Ocul Immunol Inflamm 29:1–2
Renisi G, Lombardi A, Stanzione M, Invernizzi A, Bandera A, Gori A (2021) Anterior uveitis onset after bnt162b2 vaccination: is this just a coincidence? Int J Infect Dis 110:95–97
Cunningham ET, Moorthy RS, Fraunfelder FW, Zierhut M (2019) Vaccine-associated uveitis. Ocul Immunol Inflamm 27:517–520
Steinemann TL, Koffler BH, Jennings CD (1998) Corneal allograft rejection following immunization. Am J Ophthalmol 106:575–578
Watad A, De Marco G, Mahajna H et al (2021) Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel) 9:435
Teijaro JR, Farber DL (2021) COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 21:195–197
Rodero MP, Crow YJ (2016) Type I interferon-mediated monogenic autoinflammation: the type I interferonopathies, a conceptual overview. J Exp Med 213:2527–2538
Polack FP, Thomas SJ, Kitchin N et al (2020) Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 383:2603–2615
Holloway K, Green T (2003) Drug and therapeutics committees : a practical guide. WHO Dep Essent Drugs Med Policy, Geneva, Switzerland
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception or design of the study: H.J.H. Acquisition of data for the study: S.H.E. Drafting the manuscript: S.H.E. and H.J.H.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by the Inje University Institutional Review Board (IRB no. SGP-202202010). All procedures performed in studies involving human participants were in accordance with the ethical standards of the (place name of the institution and/or national research committee) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
The requirement for informed consent was waived due to the retrospective nature of the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sim, H.E., Hwang, J.H. New onset of acute uveitis following COVID-19 vaccination. Graefes Arch Clin Exp Ophthalmol 261, 555–560 (2023). https://doi.org/10.1007/s00417-022-05798-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05798-0