Abstract
Purpose
To evaluate the functional and anatomical outcomes of a treat-and-extend (TAE) regimen with aflibercept for treatment-naive macular edema (ME) secondary to branch retinal vein occlusion (BRVO).
Methods
This was a prospective, multicenter, noncomparative, open-label clinical trial. Forty-eight eyes of 48 patients received three monthly intravitreal aflibercept injections prior to the TAE regimen. However, if the best-corrected visual acuity (BCVA) was ≥ 20/20 and the central macular thickness (CMT) was < 250 μm during the loading phase, the patient immediately proceeded to the TAE regimen. The treatment interval was adjusted by 4 weeks based on changes in CMT. The primary outcome was the mean change in BCVA from baseline to 52 weeks.
Results
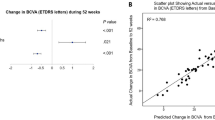
The mean change in BCVA was 23.6 ± 14.2 letters. The proportion of patients with BCVA gain ≥ 15 letters was 77.1% at 24 weeks and 72.9% at 52 weeks. The mean reduction in CMT was 326.2 ± 235.6 μm at 24 weeks and 324.2 ± 238.0 μm at 52 weeks. The mean number of injections was 6.7 ± 1.2 (range: 6–11, all patients received three monthly intravitreal aflibercept injections) over 52 weeks, and 34 patients (70.8%) reached the maximal extension interval of 16 weeks at 52 weeks.
Conclusions
The TAE regimen using aflibercept for ME secondary to BRVO, which has a treatment interval of up to 16 weeks, showed comparable efficacy to the fixed-dosing regimen along with reduced treatment burden.



Similar content being viewed by others
References
Rogers S, McIntosh RL, Cheung N et al (2010) The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 117(2):313–19. e1
Klein R, Moss SE, Meuer SM et al (2008) The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol 126(4):513–518
Rehak M, Wiedemann P (2010) Retinal vein thrombosis: pathogenesis and management. J Thromb Haemost 8(9):1886–1894
Rehak J, Rehak M (2008) Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res 33(2):111–131
Campochiaro PA, Heier JS, Feiner L et al (2010) Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 117(6):1102–12. e1
Brown DM, Campochiaro PA, Bhisitkul RB et al (2011) Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 118(8):1594–1602
Campochiaro PA, Clark WL, Boyer DS et al (2015) Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology 122(3):538–544
Clark WL, Boyer DS, Heier JS et al (2016) Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology 123(2):330–336
Holash J, Davis S, Papadopoulos N et al (2002) VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A 99(17):11393–11398
Stewart MW, Rosenfeld PJ (2008) Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol 92(5):667–668
Heier JS, Campochiaro PA, Yau L et al (2012) Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology 119(4):802–809
Campochiaro PA, Sophie R, Pearlman J et al (2014) Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. Ophthalmology 121(1):209–219
Campochiaro PA, Wykoff CC, Singer M et al (2014) Monthly versus as-needed ranibizumab injections in patients with retinal vein occlusion: the SHORE study. Ophthalmology 121(12):2432–2442
Tadayoni R, Waldstein SM, Boscia F et al (2016) Individualized stabilization criteria–driven ranibizumab versus laser in branch retinal vein occlusion: six-month results of BRIGHTER. Ophthalmology 123(6):1332–1344
Chatziralli I, Theodossiadis G, Chatzirallis A et al (2018) Ranibizumab for retinal vein occlusion: predictive factors and long-term outcomes in real-life data. Retina 38(3):559–568
Scott IU, VanVeldhuisen PC, Ip MS et al (2018) Comparison of monthly vs treat-and-extend regimens for individuals with macular edema who respond well to anti-vascular endothelial growth factor medications: secondary outcomes from the SCORE2 randomized clinical trial. JAMA Ophthalmol 136(4):337–345
Guichard M-M, Xavier AR, Türksever C et al (2018) Spectral-domain optical coherence tomography-driven treat-and-extend and pro re nata regimen in patients with macular oedema due to retinal vein occlusion: 24-month evaluation and outcome predictors. Ophthalmic Res 60(1):29–37
Yiu G, Welch RJ, Wang Y et al (2020) Spectral-domain OCT predictors of visual outcomes after ranibizumab treatment for macular edema resulting from retinal vein occlusion. Ophthalmol Retina 4(1):67–76
Sırakaya E, Küçük B, Ağadayı A (2020) Aflibercept treatment for macular edema following branch retinal vein occlusion: age-based responses. Ophthalmologica 243(2):93–100
Jaissle GB, Szurman P, Feltgen N et al (2011) Predictive factors for functional improvement after intravitreal bevacizumab therapy for macular edema due to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 249(2):183–192
Chung EJ, Hong YT, Lee SC et al (2008) Prognostic factors for visual outcome after intravitreal bevacizumab for macular edema due to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 246(9):1241–1247
de Salles MC, Amrén U, Kvanta A et al (2019) Injection frequency of aflibercept versus ranibizumab in a treat-and-extend regimen for central retinal vein occlusion: a randomized clinical trial. Retina 39(7):1370–1376
Funding
This study was funded by Bayer Korea. The sponsor provided study drug and participated in review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board (Yeungnam University Hospital IRB, Daegu, South Korea; Dong-A University Hospital IRB, Busan, South Korea; Chungnam National University Hospital IRB, Daejeon, South Korea; Maryknoll General Hospital IRB, Busan, South Korea; Chonnam National University Hospital IRB, Gwangju, South Korea), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
Author D.P. authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript. Author W.J. received a research grant from Bayer. Author J.P. received a research grant from Bayer. Author J.K. received a research grant from Bayer. Author Y.J. received a research grant from Bayer. Author M.S. received a research grant from Bayer, Novartis, and Allergan.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, DG., Jeong, W.J., Park, J.M. et al. Prospective trial of treat-and-extend regimen with aflibercept for branch retinal vein occlusion: 1-year results of the PLATON trial. Graefes Arch Clin Exp Ophthalmol 259, 2879–2886 (2021). https://doi.org/10.1007/s00417-021-05150-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05150-y