Abstract
Purpose
To identify baseline characteristics of subjects enrolled in the READ-3 study that would predict the response of macular edema to ranibizumab (RBZ) therapy at year 1.
Methods
In this post hoc analysis of the READ-3 randomized, multicenter phase 2 clinical trial, subjects with diabetic macular edema (DME) were randomized to receive monthly intravitreal injections of RBZ (0.5 or 2.0 mg) for 6 consecutive injections followed by as-needed treatments based on pre-defined retreatment criteria. In this sub-study, subjects were divided into three groups (persistent, rebound, and resolved) based on edema status at month 12 (M12). Multi-logistic regression was utilized to assess the probability of edema outcomes M12, based on the baseline characteristics.
Results
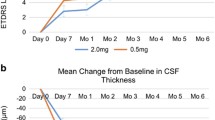
One hundred twenty-three out of 152 subjects were analyzed for this sub-study. A significant difference was observed in the baseline (BL) central subfield thickness (CST) among the study groups (p < 0.05). BL CST was a significant predictor for edema outcome at M12 with > 80% probability of the subject having persistent edema if BL CST was > 570 μm (p < 0.05). This association persisted when controlled for the dose of RBZ (relative risk (RR), 1.007; p < 0.05). BL CST was also a significant predictor for having persistent edema at M12 in subjects without vitreomacular adhesion (VMA) (> 80% probability of edema persistence at CST > 570 μm [RR, 1.006; p < 0.05]). However, in the presence of VMA, BL CST was no longer a significant predictor of having persistent edema at month 12 (RR, 1.005; p > 0.05).
Conclusions
Subjects with high CST (> 570 μm) at baseline may not benefit from repeated intravitreal injections of anti-VEGF for resolution of edema.




Similar content being viewed by others
References
Nguyen QD, Tatlipinar S, Shah SM, Haller JA, Quinlan E, Sung J, Zimmer-Galler I, Do DV, Campochiaro PA (2006) Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol 142:961–969. https://doi.org/10.1016/j.ajo.2006.06.068
Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, Abraham P, Campochiaro PA, Group R-S (2009) Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in diabetes (READ-2) study. Ophthalmology 116:2175–2181 e2171. https://doi.org/10.1016/j.ophtha.2009.04.023
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY, Meta-Analysis for Eye Disease Study G (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–564. https://doi.org/10.2337/dc11-1909
Dobrogowska DH, Lossinsky AS, Tarnawski M, Vorbrodt AW (1998) Increased blood-brain barrier permeability and endothelial abnormalities induced by vascular endothelial growth factor. J Neurocytol 27:163–173
Nicholson BP, Schachat AP (2010) A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 248:915–930. https://doi.org/10.1007/s00417-010-1315-z
Wu L, Martinez-Castellanos MA, Quiroz-Mercado H, Arevalo JF, Berrocal MH, Farah ME, Maia M, Roca JA, Rodriguez FJ, Pan American Collaborative Retina G (2008) Twelve-month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative Retina Study Group (PACORES). Graefes Arch Clin Exp Ophthalmol 246:81–87. https://doi.org/10.1007/s00417-007-0660-z
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A, group Rs (2011) The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 118:615–625. https://doi.org/10.1016/j.ophtha.2011.01.031
Channa R, Sophie R, Khwaja AA, Do DV HG, Nguyen QD, Campochiaro PA, Group R-S (2014) Factors affecting visual outcomes in patients with diabetic macular edema treated with ranibizumab. Eye (Lond) 28:269–278. https://doi.org/10.1038/eye.2013.245
Sadiq MA, Soliman MK, Sarwar S, Agarwal A, Hanout M, Demirel S, Rentiya ZS, Khan W, Do DV, Nguyen QD, Sepah YJ, Group R-S (2016) Effect of vitreomacular adhesion on treatment outcomes in the Ranibizumab for Edema of the Macula in Diabetes (READ-3) Study. Ophthalmology 123:324–329. https://doi.org/10.1016/j.ophtha.2015.09.032
Bressler SB, Ayala AR, Bressler NM, Melia M, Qin H, Ferris FL, Flaxel CJ 3rd, Friedman SM, Glassman AR, Jampol LM, Rauser ME, Diabetic Retinopathy Clinical Research N (2016) Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA Ophthalmol 134:278–285. https://doi.org/10.1001/jamaophthalmol.2015.5346
Bressler NM, Beaulieu WT, Glassman AR, Blinder KJ, Bressler SB, Jampol LM, Melia M, Wells JA 3rd, Diabetic Retinopathy Clinical Research N (2018) Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol 136:257–269. https://doi.org/10.1001/jamaophthalmol.2017.6565
Gonder JR, Walker VM, Barbeau M, Zaour N, Zachau BH, Hartje JR, Li R (2014) Costs and quality of life in diabetic macular edema: Canadian Burden of Diabetic Macular Edema Observational Study (C-REALITY). J Ophthalmol 2014:939315. https://doi.org/10.1155/2014/939315
Brown GC, Brown MM, Turpcu A, Rajput Y (2015) The cost-effectiveness of ranibizumab for the treatment of diabetic macular edema. Ophthalmology 122:1416–1425. https://doi.org/10.1016/j.ophtha.2015.03.032
Ross EL, Hutton DW, Stein JD, Bressler NM, Jampol LM, Glassman AR, Diabetic Retinopathy Clinical Research N (2016) Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment: analysis from the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial. JAMA Ophthalmol 134:888–896. https://doi.org/10.1001/jamaophthalmol.2016.1669
Do DV SYJ, Boyer D, Callanan D, Gallemore R, Bennett M, Marcus DM, Halperin L, Sadiq MA, Rajagopalan N, Campochiaro PA, Nguyen QD, Group R-S (2015) Month-6 primary outcomes of the READ-3 study (Ranibizumab for Edema of the mAcula in Diabetes-Protocol 3 with high dose). Eye (Lond) 29:1538–1544. https://doi.org/10.1038/eye.2015.142
Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M, Weichselberger A, Wolf S (2010) Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 33:2399–2405. https://doi.org/10.2337/dc10-0493
Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV BD, Heier JS, Abraham P, Thach AB, Lit ES, Foster BS, Kruger E, Dugel P, Chang T, Das A, Ciulla TA, Pollack JS, Lim JI, Eliott D, Campochiaro PA, Group R-S (2010) Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology 117:2146–2151. https://doi.org/10.1016/j.ophtha.2010.08.016
Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, Ehrlich JS, Rise, Group RR (2012) Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119:789–801. https://doi.org/10.1016/j.ophtha.2011.12.039
Sophie R, Lu N, Campochiaro PA (2015) Predictors of functional and anatomic outcomes in patients with diabetic macular edema treated with ranibizumab. Ophthalmology 122:1395–1401. https://doi.org/10.1016/j.ophtha.2015.02.036
Bressler SB, Qin H, Beck RW, Chalam KV, Kim JE, Melia M, Wells JA 3rd, Diabetic Retinopathy Clinical Research N (2012) Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol 130:1153–1161. https://doi.org/10.1001/archophthalmol.2012.1107
Sanchez-Tocino H, Alvarez-Vidal A, Maldonado MJ, Moreno-Montanes J, Garcia-Layana A (2002) Retinal thickness study with optical coherence tomography in patients with diabetes. Invest Ophthalmol Vis Sci 43:1588–1594
O'Day R, Barthelmes D, Zhu M, Wong TY, McAllister IL, Arnold JJ, Gillies MC (2013) Baseline central macular thickness predicts the need for retreatment with intravitreal triamcinolone plus laser photocoagulation for diabetic macular edema. Clin Ophthalmol 7:1565–1570. https://doi.org/10.2147/OPTH.S47424
Scott IU, Van Veldhuisen PC, Ip MS, Blodi BA, Oden NL, King J, Antoszyk AN, Peters MA, Tolentino M, Group SI (2017) Baseline factors associated with 6-month visual acuity and retinal thickness outcomes in patients with macular edema secondary to central retinal vein occlusion or hemiretinal vein occlusion: SCORE2 Study Report 4. JAMA Ophthalmol 135:639–649. https://doi.org/10.1001/jamaophthalmol.2017.1141
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and approved by a local investigational review board for some sites and by the Western Institutional Review Board for others.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Halim, M.S., Afridi, R., Hasanreisoglu, M. et al. Differences in the characteristics of subjects achieving complete, partial, or no resolution of macular edema in the READ-3 study. Graefes Arch Clin Exp Ophthalmol 259, 2941–2948 (2021). https://doi.org/10.1007/s00417-021-05148-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05148-6