Abstract
Purpose
To compare the outcomes of two different antivascular endothelial growth factor treatment regimens for treatment-naive eyes with neovascular age-related macular degeneration in routine clinical care at 12 and 24 months in Spain.
Methods
Observational study using the Fight Retinal Blindness (FRB) outcomes registry platform. Eyes were treated with fixed bimonthly (FB) aflibercept group at one center and a treat-and-extend (TAE) regimen using either aflibercept or ranibizumab at the other center.
Results
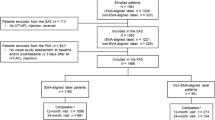
We included 192 eyes. Of these, 160 eyes (83%) completed 12 months (86 TAE and 74 FB) and 79 (41%) completed 24 months (46 for TAE and 33 for FB) of follow-up. No statistically significant differences (p > 0.05) were found regarding mean visual acuity (VA, logMAR letters) at baseline (12 month cohort TAE 59.6 vs FB 57.9; 24 month cohort TAE 61.7 vs FB 62.6), final mean VA (12 month cohort TAE 61.1 vs FB 63.0; 24 month cohort TAE 64.8 vs FB 66.4), and median number of injections (12 months TAE 7 vs FB 7; 24 months TAE 11 vs FB 12). However, the distribution of injection frequencies for the TAE group was larger, with 35% of TAE eyes receiving ≤ 6 injections at 12 months compared with only 19% of FB eyes (p = 0.024).
Conclusion
Similar VA results were observed with TAE and FB regimens, with no differences in the median number of injections. However, the TAE approach seemed to deliver a wider distribution of injection frequencies due to its individualized approach, which may help reduce the burden of injections in some eyes.


Similar content being viewed by others
Data availability
Data is available upon request to corresponding author.
References
Smith W, Assink J, Klein R et al (2001) Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 108(4):697–704
Schmidt-Erfurth U, Chong V, Loewenstein A et al (2014) Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol 98(9):1144–1167
Friedman D, O'Colmain B, Muñoz B et al (2004) Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 122(4):564–572
Rosenfeld PJ, Brown DM, Heier JS et al (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355(14):1419–1431
Brown DM, Kaiser PK, Michels M et al (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355(14):1432–1444
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF et al (2014) Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 121(1):193–201
Fung AE, Lalwani GA, Rosenfeld PJ et al (2007) An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 143(4):566–583
Martin DF, Maguire MG, Fine SL et al (2012) Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 119(7):1388–1398
Zarranz-Ventura J, Liew G, Johnston RL et al (2014) The neovascular age-related macular degeneration database: report 2: incidence, management, and visual outcomes of second treated eyes. Ophthalmology. 121(10):1966–1975
Berg K, Hadzalic E, Gjertsen I et al (2016) Ranibizumab or bevacizumab for neovascular age-related macular degeneration according to the lucentis compared to avastin study treat-and-extend protocol. Ophthalmology 123(1):51–59
Silva R, Berta A, Larsen M et al (2018) Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 125(1):57–65
DeCroos FC, Reed D, Adam MK et al (2017) Treat-and-extend therapy using aflibercept for neovascular age-related macular degeneration: a prospective clinical trial. Am J Ophthalmol 180:142–150
Almuhtaseb H, Kanavati S, Rufai SR et al (2017) One-year real-world outcomes in patients receiving fixed-dosing aflibercept for neovascular age-related macular degeneration. Eye. 31:878–883
Talks J, Daien V, Finger RP et al (2019) The use of real-world evidence for evaluating anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration. Surv Ophthalmol S0039-6257(17):30337–30335
Arnold JJ, Campain A, Barthelmes D et al (2015) Two-year outcomes of “treat and extend” intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology. 122(6):1212–1219
Jaki Mekjavić P, Gregorčič B, Oberč C et al (2018) Treat-and-extend therapy using intravitreal aflibercept for neovascular age-related macular degeneration: 2-year real-world practice data from Slovenia. BMC Ophthalmol 18(1):333
Vardarinos A, Gupta N, Janjua R et al (2017) 24-month clinical outcomes of a treat-and-extend regimen with ranibizumab for wet age-related macular degeneration in a real life setting. BMC Ophthalmol 17(1):58
Haga A, Kawaji T, Ideta R et al (2018) Treat-and-extend versus every-other-month regimens with aflibercept in age-related macular degeneration. Acta Ophthalmol 96(3):e393–e3e8
Gillies MC, Walton RJ, Arnold JJ et al (2014) Comparison of outcomes from a phase 3 study of age-related macular degeneration with a matched, observational cohort. Ophthalmology. 121(3):676–681
Mehta H, Tufail A, Daien V et al (2018) Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res 65:127–146
Gillies M, Walton R, Liong J et al (2014) Efficient capture of high-quality data on outcomes of treatment for macular diseases: the Fight Retinal Blindness! Project. Retina. 34:188–195
Wong WL, Su X, Li X et al (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2(2):e106–e116
Gillies MC, Hunyor AP, Arnold JJ et al (2019) Effect of ranibizumab and aflibercept on best-corrected visual acuity in treat-and-extend for neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol 137(4):372–379
Ohji M, Takahashi K, Okada AA et al (2020) Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR. Adv Ther 37(3):1173–1187
Barthelmes D, Nguyen V, Daien V et al (2018) Two year outcomes of “Treat and Extend” intravitreal therapy using aflibercept preferentially for neovascular age-related macular degeneration. Retina. 38(1):20–28
Gillies MC, Campain A, Barthelmes D et al (2015) Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 122(9):1837–1845
Guymer RH, Markey CM, McAllister IL et al (2018) Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology. 126(5):723–734
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MCG and DB are inventors of the software used to collect the data for this analysis. MFR reports personal fees from Allergan outside the submitted work. JZV reports personal fees from Allergan, Bayer, Novartis, and Roche outside the submitted work.
Ethics approval
Institutional review board and ethics committee approvals were obtained from both study sites.
Consent to participate and for publication
All study participants provided written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Figueras-Roca, M., Parrado-Carrillo, A., Nguyen, V. et al. Treat-and-extend versus fixed bimonthly treatment regimens for treatment-naive neovascular age–related macular degeneration: real world data from the Fight Retinal Blindness registry. Graefes Arch Clin Exp Ophthalmol 259, 1463–1470 (2021). https://doi.org/10.1007/s00417-020-05016-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-05016-9