Abstract
Purpose
To evaluate 24-week visual acuity and anatomic outcomes of two “pro re nata” (prn) treatment strategies (intravitreal bevacizumab [IVB] prn versus intravitreal triamcinolone acetonide [IVT] prn) in patients with persistent diabetic macular edema (pDME) after 24 weeks of prn-IVB.
Methods
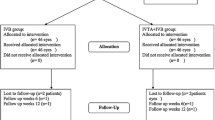
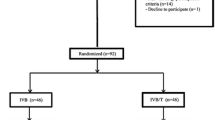
One hundred eyes with center-involving DME were enrolled and treated with prn-IVB for 24 weeks; at week 24, eyes with pDME (central subfield thickness [CST] on spectral domain optical coherence tomography > 300 μm) were randomized to IVB monthly prn (group I; prn-IVB) or IVT every 3 months prn (group II; prn-IVT) and eyes in which the CST was ≤ 300 μm were assigned to continue prn-IVB (group III).
Results
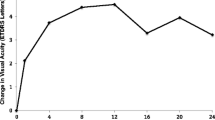
Seventy-four eyes completed a 48-week study period. At week 24, 65 (79.3%) eyes still had DME with CST > 300 μm and, therefore, were randomized to prn-IVB (group I, n = 33) or prn-IVT (group II, n = 32); the remaining 17 (20.7%) eyes had CST ≤ 300 μm and were assigned to continued treatment with prn-IVB (group III). At baseline, mean CST (μm) ± standard error of the mean (SEM) was 447.2 ± 24.4, 478.0 ± 19.7, and 386.0 ± 21.0 in groups I, II, and III, respectively (p > 0.05). At week 48, there was no significant difference in mean CST between groups I and II (369.9 ± 23.3 and 426.0 ± 26.1, respectively; p = 0.9995). A significant reduction in mean CST, compared with baseline, was noted at weeks 28 (p = 0.0002) and 44 (p = 0.0002) in group II. Group I did not show a significant reduction in mean CST compared with baseline at any study visit. There were no significant differences in mean CST between groups I and II at any study visit. At baseline, mean ± SEM best-corrected visual acuity (BCVA) (logMAR) was 0.50 ± 0.00, 0.60 ± 0.10, and 0.50 ± 0.10 in groups I, II, and III, respectively (p > 0.05). At week 48, there was no statistically significant difference in mean BCVA between groups I and II (0.50 ± 0.10 and 0.80 ± 0.10, respectively; p = 0.4473). There was no significant improvement in mean BCVA, as compared with baseline, at any study follow-up visit in any of the groups. Group II demonstrated significantly lower BCVA after 24 weeks of IVT (at week 48) compared with baseline (p = 0.0435). There was no significant difference in mean BCVA between groups I and II at any time-point.
Conclusion
In eyes with pDME after 24 weeks of treatment with prn-IVB, there was no difference between continued treatment with prn-IVB versus a treatment switch to prn-IVT with respect to mean BCVA or mean CST at week 48. However, BCVA was stable in the prn-IVB group, while prn-IVT was associated with BCVA reduction from baseline and a higher risk of IOP elevation.





Similar content being viewed by others
References
Federation ID(2017) IDF diabetes, 8 ed. https://diabetesatlas.org/resources/2017-atlas.html. Accessed 7 May 2019
Yau JW, Rogers SL, Kawasaki R et al (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35(3):556–564
Das A, McGuire PG, Rangasamy S (2015) Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology 122(7):1375–1394
Elman MJ, Aiello LP, Beck RW et al (2010) Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 117(6):1064–1077.e35
Wells JA, Glassman AR, Ayala AR et al (2015) Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 372(13):1193–1203
Channa R, Sophie R, Khwaja AA et al (2013) Factors affecting visual outcomes in patients with diabetic macular edema treated with ranibizumab. Eye (Lond) 28(3):269–278
Brown DM, Nguyen QD, Marcus DM et al (2013) Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 120(10):2013–2022
Elman MJ, Bressler NM, Qin H et al (2011) Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 118(4):609–614
Bressler SB, Ayala AR, Bressler NM et al (2016) Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA Ophthalmol 134(3):278–285
Nauck M, Karakiulakis G, Perruchoud AP et al (1998) Corticosteriods inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol 341(2–3):309–315
Nauck M, Roth M, Tamm M et al (1997) Induction of vascular endothelial growth factor by platelet-activating factor and platelet-derived growth factor is downregulated by corticosteroids. Am J Respir Cell Mol Biol 16(4):398–406
Mason JO 3rd, White MF, Feist RM et al (2008) Incidence of acute onset endophthalmitis following intravitreal bevacizumab injection. Retina 28(4):564–567
Aiello LP, Brucker AJ, Chang S, et al.(2004) Evolving guidelines for intravitreal injections. Retina; 24(5 Suppl):S3–19
Hauser D, Bukelman A, Pokroy R et al (2008) Intravitreal triamcinolone for diabetic macular edema: comparison of 1, 2, and 4 mg. Retina 28(6):825–830
Michaelides M, Kaines A, Hamilton RD et al (2010) A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology 117(6):1078–1086
Elman MJ, Qin H, Aiello LP et al (2012) Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment. Diabetic Retinopathy Clinical Research Network* Writing Committee: Ophthalmology 119(11):2312–2318
Aiello LP, Beck RW, Bressler NM, et al.(2011) Rationale for the diabetic retinopathy clinical research network protocol for center-involved diabetic macular edema. diabetic retinopathy clinical research network. Writing Committee: Ophthalmology; 118(12):e5–14
Willmann G, Nepomuceno AB, Messias K et al (2017) Foveal thickness reduction after anti-VEGF treatment in chronic diabetic macular edema. Int J Ophthalmol 10(5):760–764
Wang MZ, Feng K, Lu Y et al (2016) Predictors of short-term outcomes related to central subfield foveal thickness after intravitreal bevacizumab for macular edema due to central retinal vein occlusion. Int J Ophthalmol 9(1):86–92
Paccola L, Costa RA, Folgosa MS et al (2008) Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular edema (IBEME study). Br J Ophthalmol 92(1):76–80
Lee J, Moon BG, Cho AR et al (2016) Optical coherence tomography angiography of DME and its association with anti-VEGF treatment response. Ophthalmology 123(11):2368–2375
Bressler SB, Odia I et al (2019) Factors associated with visual acuity and central subfield thickness changes when treating diabetic macular edema with anti-vascular endothelial growth factor therapy: an exploratory analysis of the protocol t randomized clinical trial. JAMA Ophthalmology 137(4):382–389
Kreutzer TC, Al Saeidi R, Kook D, et al.(2010) Comparison of intravitreal bevacizumab versus triamcinolone for the treatment of diffuse diabetic macular edema. Department of Ophthalmology, Ludwig-Maximilians University, Munich, Germany. Ophthalmologica; 224(4):258–264
Penha FM, Maia M, Cardillo JA, Arevalo JF, Wu L, Rodriguez FJ, Berrocal MH, Farah ME, The Pan-American Collaborative Retina Study Group (PACORES) (2012) Comparison of a single intravitreal injection of bevacizumab versus triamcinolone acetonide as primary treatment for diffuse diabetic macular oedema. Acta Ophthalmol 90(2):e160–e161
Neto HO, Regatieri CV, Nobrega MJ et al (2017) Multicenter, randomized clinical trial to assess the effectiveness of Intravitreal injections of bevacizumab, triamcinolone, or their combination in the treatment of diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina 48(9):734–740
Otani T, Yamaguchi Y, Kishi S (2010) Correlation between visual acuity and foveal microstructural changes in diabetic macular edema. Retina 30(5):774–780
Alasil T, Keane PA, Updike JF et al (2010) Relationship between optical coherence tomography retinal parameters and visual acuity in diabetic macular edema. Ophthalmology 117(12):2379–2386
Das R, Spence G, Hogg RE et al (2018) Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmol 136(2):202–208
Liu S, Wang D, Chen F et al (2019) Hyperreflective foci in OCT image as a biomarker of poor prognosis in diabetic macular edemapatients treating with Conbercept in China. BMC Ophthalmol 19(1):157
Jonas JB, Degenring RF, Kreissig I et al (2005) Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology 112(4):593–598
Puliafito CA, Cousins SW, Bacharach J et al (2016) Forming a consensus: data and guidance for physicians treating diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina 47(4):S4–S15
Maturi RK, Glassman AR, Liu D et al (2018) Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema: a DRCR network phase 2 randomized clinical trial. JAMA Ophthalmol 136(1):29–38
Lang GE, Berta A, Eldem BM, Simader C, Sharp D, Holz FG, Sutter F, Gerstner O, Mitchell P, RESTORE Extension Study Group (2013) Two-year safety and efficacy of ranibizumab 0.5 mg in diabetic macular edema: interim analysis of the RESTORE extension study. Ophthalmology. 120(10):2004–2012
CATT Research Group, Martin DF, Maguire MG, Ying GS et al (2011) Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 364(20):1897–1908
Elman MJ, Ayala A, Bressler NM et al (2015) Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology 122(2):375–381
Michalska-Małecka K, Kabiesz A, Kimsa MW et al (2016) Effects of intravitreal ranibizumab on the untreated eye and systemic gene expression profile in age-related macular degeneration. Clin Interv Aging 11:357–365
Matsuyama K, Ogata N, Matsuoka M et al (2011) Effects of intravitreally injected bevacizumab on vascular endothelial growth factor in fellow eyes. J Ocul Pharmacol Ther 27(4):379–383
Acknowledgments
We thank Ms. Lucélia Albieri (ophthalmic technician and patient advisor) from University of São Paulo (Brazil) for image acquisition and patient counseling.
Contributors
JAC and RJ are the primary contributors to the research design. MWR is responsible for research execution and data acquisition. AM is the primary contributor to data analysis. Manuscript preparation by MWR with revisions and interpretation was provided by JAC, RCS, IUS, and RJ.
Funding
The project had financial support by CNPq government (National Research Council) grant number 142177/2016-4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ribeirão Preto Medical School at University of São Paulo and with the 1964 Declaration of Helsinki and its later amendments.
Statement of informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
S. Figure 1.
Diamond graphs merge the mean distribution of number injections in each group (I, II and III) after 48 weeks of treatment. The individual total number of injections was higher (P<0.001) in group I: (mean ± SE) 11.3 ± 0.4 than in group II: 8.2 ± 0.3, and both were greater than (P<0.001) in group III: 5.1 ± 0.8 (ANOVA followed by all pairs Tukey-Kramer HSD). (JP2 785 kb)
S. Figure 2.
Optical coherence tomography from Group I patients at Baseline, 24-week and 48-week study periods. Different architectural changes were verified. CME was verified in Cases N2, N7, N16 and N23. Cases N2 and N7 presented DRIL. Hyperrefllective plaques were found in case N7. Hyperreflective foci were seen in cases N2, N7, N23 and N42. Ellipsoid/ELM attenuation was seen in cases N2, N7, N16, N23. Progressive SRD was presented in case N16. (JP2 940 kb)
S. Figure 3.
Optical coherence tomography from Group II patients at Baseline, 24-week and 48-week study periods. Different architectural changes were verified. CME was verified in Cases N22, N24, N33, N35 and N44. Case N1 presented DRIL. Hyperrefllective foci and/or exsudates were found in cases N1, N24, N33, N34, N35 and N44. Ellipsoid/ELM attenuation was seen in cases N22, N24, N33, N34, N35 and N44. SRD were presented in cases N22, N24, N33, N34 and N35. Case N44 presented progressive SRD. (JP2 457 kb)
S. Figure 4.
Optical coherence tomography from Group I patients at Baseline, 24-week and 48-week study periods. Different architectural changes were verified. CME was verified in Cases N28, N38, and N40. Cases N13 and N26 presented DRIL. Hyperrefllective foci and/or exsudates were found in cases N13 and N26. Hyperrefllective plaque was showed in case N26. Ellipsoid/ELM attenuation was seen in cases N26 and N40. (JP2 510 kb)
S. Table 1.
Number of injections. (DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Rodrigues, M.W., Cardillo, J.A., Messias, A. et al. Bevacizumab versus triamcinolone for persistent diabetic macular edema: a randomized clinical trial. Graefes Arch Clin Exp Ophthalmol 258, 479–490 (2020). https://doi.org/10.1007/s00417-019-04564-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04564-z