Abstract
Purpose
To determine the predictive factors for recurrent macular edema due to branch retinal vein occlusion (BRVO) during intravitreal ranibizumab (IVR) monotherapy.
Methods
Clinical records were retrospectively reviewed for 65 patients (mean age 66.5 years, 65 eyes) who were diagnosed with macular edema due to BRVO and treated with IVR monotherapy for 12 months at the Medical Retina Division, Department of Ophthalmology, Keio University Hospital between October 2013 and August 2017. Best-corrected visual acuity (BCVA), fundus findings, and sectional optical coherence tomography (OCT) images were analyzed.
Results
Overall BCVA and central retinal thickness (CRT) improved (all p < 0.01). BCVA at 12 months was significantly worse in patients with recurrent macular edema (40 eyes [61.5%]) (p < 0.01) than in those without, while CRT decreased and was comparable in both groups at 12 months. Logistic regression analyses showed association of recurrence with disorganization of the retinal inner layer (DRIL) temporal to the fovea at baseline (odds ratio = 7.74; 95% confidence interval 1.62–37.08, p = 0.01), after adjusting for age, gender, and initial CRT.
Conclusion
Recurrent macular edema due to BRVO affects visual outcome and is associated with initial DRIL temporal to the fovea, evaluated using OCT sectional images before treatments. DRIL may facilitate determination of follow-up schedules in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Branch retinal vein occlusion (BRVO) is a common retinal disease [1,2,3] that causes sudden impairment of visual function. In several clinical studies, anti-vascular endothelial growth factor (VEGF) therapy has been used to rapidly reduce macular edema [4,5,6,7,8,9]. This condition can be naturally resolved over a period of 2 years in approximately 80% of patients [10], although the visual outcomes may not be as good as those in treated eyes considering the neurodegeneration that occurs due to prolonged edema. Macular edema decreases as early as 1 week after the initial treatment, and best-corrected visual acuity (BCVA) improves in response to this anatomical recovery [4,5,6,7,8]. However, recurrent macular edema may occur, which should be treated as soon as possible to protect the retinal neural elements and obtain better visual outcome.
Clinicians have used a pro re nata (PRN) regimen for anti-VEGF treatment of macular edema due to BRVO [11,12,13]. Thus, recurrences of macular edema must be detected early to ensure that appropriate additional treatments are delivered. Monthly follow-up visits may be helpful in this regard; however, it is not practical to implement this schedule in all patients with BRVO. Instead, it would be useful to determine which patients should receive frequent follow-up or be treated with a treat and extend regimen to avoid recurrent macular edema. Researchers must therefore develop methods to predict recurrence of macular edema due to BRVO.
Previous studies have shown that decreased macular vessel density, as determined with optical coherence tomography angiography (OCTA) [11] and fluorescein angiography (FA) [14], is related to recurrence after anti-VEGF treatment. However, before treatment, it may be difficult to perform OCTA and FA because massive retinal hemorrhage and/or edema in patients with fresh BRVO can cause segmentation error and fluorescence blockade, respectively.
In contrast, sectional images of the retina can easily be obtained using OCT. In this regard, previous reports have focused on findings from the external limiting membrane (ELM), ellipsoid zone (EZ), and interdigitation zone (IZ) as predictors of visual outcome after macular edema due to BRVO [15,16,17,18]. However, the predictive factors for recurrences have remained unclear.
Because BRVO lesions originate in the inner retinal layers, it may be useful to measure the impact of inner retinal condition with sectional OCT images. A recent report stated that disorganization of the retinal inner layer (DRIL) may influence improvement of visual acuity in patients with central retinal vein occlusion (CRVO) or BRVO after 6 intravitreal injections of bevacizumab delivered monthly [19]. Conversely, 2 additional reports have shown that DRIL is not related to visual outcome in BRVO [20, 21]. However, these prior studies did not assess recurrence. DRIL was originally reported as a biomarker for use in predicting the prognosis of diabetic macular edema in terms of visual acuity [22, 23] and was later reported to be related to abnormal blood flow [24, 25]. Recurrence of macular edema can theoretically occur in relation to the vulnerability of the blood vessels. Thus, we hypothesized that factors related to abnormal blood flow may predict the risk of recurrent macular edema.
In the present study, we first analyzed the influence of recurrent macular edema on visual acuity to determine the impact of recurrence predictors, then, we analyzed factors that may be predictive of recurrence by using retinal sections obtained with OCT at baseline (prior to treatment). Information regarding these predictive factors may be useful for clinicians in managing macular edema due to BRVO.
Materials and methods
This retrospective study was based on a detailed medical chart review; it followed the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Keio University School of Medicine. All patients provided informed consent for the use of their data for research purposes.
Study participants
In total, 117 consecutive eyes of 117 patients were diagnosed with BRVO. From these, we included in the present study 65 eyes of 65 patients with BRVO-induced macular edema who had received intravitreal ranibizumab (IVR) monotherapy at the Medical Retina Division Clinic of the Department of Ophthalmology, Keio University Hospital (Tokyo, Japan) between October 2013 and August 2017. All patients had attended our clinic for at least 12 months, during which no treatment other than IVR was administered. Patients were excluded if they had a history of any anti-VEGF treatment (10 patients) or photocoagulation (12 patients), regardless of both with or without corticosteroid use within 6 months prior to the initial IVR injection. Those who received corticosteroid and/or photocoagulation therapy (12 patients), who had been sent back to the local clinicians (2 patients), as well as those who gave up or denied IVR upon recurrence for patient-specific reasons (7 patients), and who defaulted visits were lost to follow-up (9 patients) within 12 months after the first IVR treatment were also excluded.
Ophthalmologic examinations
At each follow-up visit, all patients underwent BCVA measurement using refraction tests, slit-lamp examinations, and binocular indirect ophthalmoscopy—ophthalmoscopy was performed after pupil dilation with 0.5% tropicamide. BCVA improvement or worsening was defined as a change of > 0.2 in LogMAR.
FA
FA was performed and fundus photographs were obtained using a Topcon TRC 50DX retinal camera (Topcon Corporation, Tokyo, Japan) to diagnose BRVO and macular edema and to evaluate foveal hemorrhage and distance from disc to occlusive lesion.
OCT
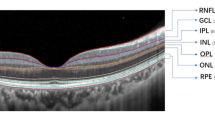
OCT images were recorded at every follow-up visit using a Heidelberg Spectralis OCT system (Heidelberg Engineering GmbH, Dossenheim, Germany). The images were used to evaluate the central retinal thickness (CRT), central choroidal thickness (CCT), DRIL, subretinal fluid build-up, macular edema, and disruptions of ELM and EZ. CRT was defined as the distance between the internal limiting membrane and the putative retinal pigment epithelium at the fovea. CCT was defined as the distance from the hyper-reflective line corresponding to Bruch’s membrane beneath the retinal pigment epithelium and the inner surface of the sclera at the foveal center. DRIL was identified when the boundaries between the ganglion cell layer and inner plexiform layer complex, inner nuclear layer, and outer plexiform layer (OPL) could not be distinguished, as described in a previous report [25]. DRIL within 500 μm of the fovea in nasal, temporal, superior, and inferior directions was defined in respective horizontal and vertical OCT images (Fig. 1a–c). Measurements were performed using the caliper function of the OCT device, with manual adjustment. Image analyses were performed by 2 retina specialists (MS and YO) in a blinded manner; if the results differed between the 2 specialists, a 3rd specialist (NN) was asked to perform analysis and results were determined by consensus.
Disorganization of the retinal inner layer (DRIL) in optical coherence tomography (OCT) images in a representative eye. DRIL was analyzed within 500 μm of the fovea in both the vertical and horizontal directions (a). OCT images (b, horizontal section; c, vertical section) of a left eye with macular edema due to branch retinal vein occlusion at baseline. DRIL was observed temporal to the fovea within 500 μm of the fovea (bi-arrows). However, it was not observed on other sides of the fovea
Intravitreal ranibizumab monotherapy and follow-up
During the induction phase of the treatment, ranibizumab (0.5 mg, 0.05 mL) was injected intravitreally via the pars plana under sterile conditions. This injection was then repeated as needed (PRN) if follow-up OCT showed macular edema. The need for retreatment was determined by retinal specialists (MS, NN, HS, and YO). Follow-ups were conducted every 1 month after therapy initiation until the macular edema disappeared with the PRN regimen of anti-VEGF therapy. When no macular edema was detected for more than 2 months, the follow-up interval was extended to 2 months.
Statistical analyses
Data are expressed as mean ± standard deviation. Commercially available software (IBM SPSS Statistics, version 24.0; IBM Japan, Tokyo, Japan) was used for all statistical analyses. The Wilcoxon signed-rank test, Mann–Whitney U test, chi-squared test, and logistic regression analyses were used. All p values < 0.05 were considered to be statistically significant.
Results
Sixty-five eyes of 65 patients (male, 33 eyes [50.8%]; mean age, 66.5 years; Table 1) were included. The mean number of injections was 1.9 ± 1.1 (range 1–5) for the initial resolution of macular edema and 3.5 ± 1.8 (range 1–7) over the 12-month follow-up period. Eleven eyes (16.9%) required no additional injections during the 12-month follow-up period after the initial injection. Recurrence of macular edema after remission was observed in 40 eyes (61.5%) during the 12-month follow-up period (Table 2).
Overall, the mean BCVA (Fig. 2a) and CRT (Fig. 2b) were improved at 3, 6, and 12 months, compared with the baseline values (p < 0.01 for all); this improvement was observed regardless of the presence of recurrent macular edema after initial resolution of macular edema (Fig. 2a, b, p < 0.01 for all). BCVA improved or was maintained in 62 eyes (95.4%) after 12 months (data not shown).
Mean best-corrected visual acuity (BCVA) and central retinal thickness (CRT) at each time point. Mean ± standard deviation of the BCVA (a) and CRT (b) at baseline and at 3, 6, and 12 months after initial intravitreal ranibizumab therapy with PRN regimen. Overall, the mean BCVA (a) and CRT (b) were improved at 3, 6, and 12 months, compared with baseline values, in the recurrent and non-recurrent groups by Wilcoxon signed-rank test (**p < 0.01 for all). Mean BCVA did not differ at baseline between the 2 groups; however, the values at 3, 6, and 12 months after therapy were worse in the recurrent group than in non-recurrent group, as determined by the Mann–Whitney U test (a, ††p < 0.01 for all). Mean CRT was greater in the recurrent group at baseline and at 3 months by Mann–Whitney U test (b, ††p < 0.01 for both); however, at 6 and 12 months, it was comparable between the groups
Interestingly, when the mean values between the groups with and without recurrence were compared, mean BCVA at each time point after treatment, including at the 12-month follow-up, was worse in patients who had recurrent macular edema (Fig. 2a, p < 0.01 for all), whereas the initial mean BCVA was nearly identical in both groups (Fig. 2a, Table 2, p = 0.396). Mean CRT was different between the groups at baseline (Table 2, p < 0.001) and at the 3-month follow-up (Fig. 2b, p < 0.01); however, the values became comparable at the 6-month follow-up, with additional injections as needed (Fig. 2b).
Differences in the initial conditions of the eyes with and without recurrence are shown in Table 2. In eyes with recurrence, the mean age of the patients was older (68.8 ± 10.0 years vs. 62.7 ± 9.6 years; p = 0.01); the mean CRT was greater (563.3 ± 194.3 μm vs. 380.2 ± 117.2 μm; p < 0.001); and the incidences of DRIL nasal (73% vs. 28%; p < 0.001), inferior (73% vs. 32%; p = 0.001), and temporal to (80% vs. 24%; p < 0.001) the fovea were higher, relative to those in eyes without recurrence. The incidences of disruptions of ELM and EZ did not differ between eyes with and without recurrence. The total number of injections required during the 12-month follow-up period was significantly greater in eyes with recurrence (Table 2, 4.4 ± 1.6 vs. 2.0 ± 1.1 injections, respectively; p < 0.001). The mean duration to the first recurrence after the first remission was 4.4 ± 5.8 months (data not shown). The incidence of DRIL temporal to the fovea was also higher at the time of first macular edema remission and at the 12-month follow-up in eyes with recurrence (Supplementary Table 1).
Next, the risk of recurrence was analyzed using logistic regression analysis (Table 3). Crude analysis showed that increased age (p = 0.01); increased CRT (p = 0.001); and eyes with DRIL nasal (p = 0.002), inferior (p = 0.004), and temporal (p < 0.001) to the fovea initially exhibited increased risk for recurrence of macular edema. Moreover, after adjustment for age, gender, and initial CRT at baseline, eyes with DRIL temporal to the fovea at baseline exhibited a significantly greater risk of recurrence (odds ratio = 7.74, 95% confidence interval 1.62–37.08, p = 0.01), indicating that DRIL temporal to the fovea was an independent risk factor for recurrent macular edema.
Discussion
In the present study, we showed that, in eyes with macular edema due to BRVO, both BCVA and CRT were improved by IVR monotherapy after 12 months, using a regimen of 1 initial injection followed by a PRN approach. However, recurrent macular edema occurred in 61.5% of the eyes; recurrence affected BCVA at the 12-month follow-up. The initial CRT was greater in the group with recurrent macular edema; however, CRT decreased and became comparable to that in the non-recurrent group with treatment. Presence of DRIL temporal to the fovea was an independent risk factor for recurrent macular edema due to BRVO, as shown by logistic regression analysis after adjustment for age, gender, and initial CRT.
Both mean BCVA and CRT were improved rapidly 3 months after the initial IVR injection, corroborating previous reports of prospective clinical trials using either ranibizumab or aflibercept injection [6, 26]. In these trials, 6 injections were delivered at monthly intervals, followed by PRN treatment thereafter. The current study observed similar outcomes with only 1 injection followed by a PRN regimen. In addition, none of the patients in the current study required 6 consecutive injections to initiate therapy until the first remission of macular edema, with monthly follow-up until the first remission (data not shown).
The mean number of injections delivered in the current study was 3.5, corroborating the findings of a previous study by Miwa et al. [27], which showed that 3.8 injections were required, using the same protocol for PRN treatment. Macular edema recurred in 61.5% of patients in the current study, which was consistent with the results of a previous study by Hasegawa et al. [11] (59.3%); they reported respective injection numbers of 5.1 and 2.1 in eyes with and without recurrence, which were also consistent with the findings of the present study.
Notably, the visual outcome was worse in eyes with recurrent macular edema, although CRT was successfully reduced to levels similar to those in the non-recurrent group. However, retinal neurons may have been more severely affected by changes in the microenvironment, such as cytokine and ionic changes, related to repeated macular edema over a prolonged period. Although VEGF plays a neuroprotective role [28, 29], the extracellular fluid that causes edema may contain various neurotoxic cytokines that can damage the retinal neurons; previous reports have shown that levels of various inflammatory cytokines (e.g., interleukin-6 and monocyte chemotactic protein-1) were increased in the vitreous fluid of BRVO patients [30].
Alternative or additional pathways may contribute to visual outcomes. Previous studies regarding visual outcomes have focused on the outer layers of the retina, such as the ELM, EZ, and IZ [15,16,17,18, 20]. In contrast, there were no significant differences between the recurrence of macular edema and the incidences of ELM or EZ disruption in the current study. Nevertheless, eyes with recurrent macular edema had worse visual outcomes, likely because recurrence was associated with DRIL, which is reportedly related to visual function, as demonstrated in diabetic retinopathy patients [22]. DRIL represents a disorganized microenvironment involving synapses between photoreceptors and secondary neurons (bipolar cells) that construct the OPL. In addition, a persistent synaptic abnormality may cause retrograde damage to photoreceptors. Indeed, this idea is supported by the observation that visual prognosis is related to the flow in the deep capillary plexus of the inner retinal layer [31, 32]. Another previous report stated that non-perfused areas in the inner layer, which can be detected by FA, correspond to EZ disruption that reflects photoreceptor damage [33]. Thus, abnormal inner retinal blood flow and recurrent macular edema may cause damage to photoreceptors. Analysis of a hyper-reflective band involving the inner retinal layer in OCT images, known as paracentral acute middle maculopathy (PAMM), showed that ischemic lesions in vascular occlusive diseases of the retina develop at the venular pole of the deep capillary plexus [34] and may then progress laterally and anteriorly into the inner retina at the level of the superficial capillary plexus. This ischemic mechanism also supports our abovementioned hypothesis that inner retinal findings involve photoreceptor disorder.
Importantly, recurrence was associated with the presence of DRIL temporal to the fovea, but not with the disruption of either ELM or EZ. Given that DRIL is related to flow abnormality in the deep plexus [24], the areas affected by DRIL may have experienced persistent hypoxia, leading to excessive VEGF expression and thus recurrent macular edema. Alternatively, hypoxia may have interrupted the recovery of vessels that were influenced at the time of occlusion due to insufficient nutrient and oxygen supply to the affected vascular cells. This interpretation of our results is consistent with a previous report using FA, which found that 35% of patients showed recurrence 6 months after a single injection of an anti-VEGF drug; the risk was increased if the patients had a non-perfused area in the deep capillary plexus within 1 mm of the fovea [14]. Notably, the previous study using FA had a limitation in the recording of clear images if the eyes had severe hemorrhage causing blockade of the signals [14]. In contrast, the current study did not have this limitation; the inner retinal layers in the sectional OCT images could be evaluated regardless of the extent of hemorrhage and edema before treatment. DRIL, as identified in sectional OCT images, would be of value for predicting recurrent macular edema in daily clinical practice.
DRIL temporal to the fovea was responsible for the recurrence of macular edema. Notably, retinal vessels temporal to the fovea are at a greater distance from the disc, and the presence of microvascular changes on the temporal side may result in a greater area of vascular abnormality. This would increase the risk of extracellular fluid accumulation. Alternatively, because the capillary densities in the neurofiber layer, ganglion cell layer, inner plexiform layer, and inner nuclear layer are lower on the side temporal to the fovea [35], we speculate that further reductions in blood flow caused by BRVO, as suggested by DRIL, could lower the ability of the retina to absorb extracellular fluid, thereby resulting in edema.
The limitations of the current study are its relatively small sample size and retrospective design; moreover, the need for retreatment was determined by attending doctors, rather than by the reading center. However, recurrent macular edema was recorded in OCT images that were reviewed during the current study, and we confirmed that retreatment was performed properly, based on the criterion, if follow-up OCT showed macular edema.
In summary, visual prognosis was worse in eyes with recurrent macular edema, despite reduction in CRT that indicated sufficient treatment. DRIL temporal to the fovea was a significant risk factor for recurrence. Use of OCT sectional images is valuable for diagnosis of macular edema, as well as for evaluation of DRIL temporal to the fovea at baseline; this can help to predict prognosis and determine follow-up schedules for early retreatment of recurrent edema and/or the treatment protocol in the daily clinic.
References
Rogers SL, McIntosh RL, Lim L, Mitchell P, Cheung N, Kowalski JW et al (2010) Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology 117(6):1094–1101
Yasuda M, Kiyohara Y, Arakawa S, Hata Y, Yonemoto K, Doi Y et al (2010) Prevalence and systemic risk factors for retinal vein occlusion in a general Japanese population: the Hisayama study. Invest Ophthalmol Vis Sci. 51(6):3205–3209
Mitchell P, Smith W, Chang A (1996) Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 114(10):1243–1247
Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N et al (2010) Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 117(6):1124–1133 e1
Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N et al (2011) Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology 118(10):2041–2049
Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC et al (2010) Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 117(6):1102–1112 e1
Ehlers JP, Kim SJ, Yeh S, Thorne JE, Mruthyunjaya P, Schoenberger SD et al (2017) Therapies for macular edema associated with branch retinal vein occlusion: a report by the American Academy of Ophthalmology. Ophthalmology 124(9):1412–1423
Heier JS, Campochiaro PA, Yau L, Li Z, Saroj N, Rubio RG et al (2012) Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology 119(4):802–809
Clark WL, Boyer DS, Heier JS, Brown DM, Haller JA, Vitti R et al (2016) Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology 123(2):330–336
Hayreh SS, Zimmerman MB (2014) Branch retinal vein occlusion: natural history of visual outcome. JAMA Ophthalmol. 132(1):13–22
Hasegawa T, Murakawa S, Maruko I, Kogure-Katakura A, Iida T (2018) Correlation between reduction in macular vessel density and frequency of intravitreal ranibizumab for macular oedema in eyes with branch retinal vein occlusion. Br J Ophthalmol. 103(1):72–77
Shiono A, Kogo J, Sasaki H, Yomoda R, Jujo T, Tokuda N et al (2018) Optical coherence tomography findings as a predictor of clinical course in patients with branch retinal vein occlusion treated with ranibizumab. PLoS One. 13(6):e0199552
Tilgner E, Dalcegio Favretto M, Tuisl M, Wiedemann P, Rehak M (2017) Macular cystic changes as predictive factor for the recurrence of macular oedema in branch retinal vein occlusion. Acta Ophthalmol. 95(7):e592–e5e6
Yoo JH, Ahn J, Oh J, Cha J, Kim SW (2017) Risk factors of recurrence of macular oedema associated with branch retinal vein occlusion after intravitreal bevacizumab injection. Br J Ophthalmol. 101(10):1334–1339
Kang HM, Chung EJ, Kim YM, Koh HJ (2013) Spectral-domain optical coherence tomography (SD-OCT) patterns and response to intravitreal bevacizumab therapy in macular edema associated with branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 251(2):501–508
Moon BG, Cho AR, Kim YN, Kim JG (2018) Predictors of refractory macular edema after branch retinal vein occlusion following intravitreal bevacizumab. Retina. 38(6):1166–1174
Murakami T, Okamoto F, Iida M, Sugiura Y, Okamoto Y, Hiraoka T et al (2016) Relationship between metamorphopsia and foveal microstructure in patients with branch retinal vein occlusion and cystoid macular edema. Graefes Arch Clin Exp Ophthalmol. 254(11):2191–2196
Muraoka Y, Tsujikawa A, Takahashi A, Iida Y, Murakami T, Ooto S et al (2015) Foveal damage due to subfoveal hemorrhage associated with branch retinal vein occlusion. PLoS One. 10(12):e0144894
Mimouni M, Segev O, Dori D, Geffen N, Flores V, Segal O (2017) Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with macular edema secondary to vein occlusion. Am J Ophthalmol. 182:160–167
Nakano E, Ota T, Jingami Y, Nakata I, Hayashi H, Yamashiro K (2018) Disorganization of the retinal inner layers after anti-VEGF treatment for macular edema due to branch retinal vein occlusion. Ophthalmologica 240(4):229–234
Babiuch AS, Han M, Conti FF, Wai K, Silva FQ, Singh RP (2019) Association of disorganization of retinal inner layers with visual acuity response to anti-vascular endothelial growth factor therapy for macular edema secondary to retinal vein occlusion. JAMA Ophthalmol. 137(1):38–46
Sun JK, Radwan SH, Soliman AZ, Lammer J, Lin MM, Prager SG et al (2015) Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes 64(7):2560–2570
Sun JK, Lin MM, Lammer J, Prager S, Sarangi R, Silva PS et al (2014) Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 132(11):1309–1316
Spaide RF (2015) Volume-rendered optical coherence tomography of diabetic retinopathy pilot study. Am J Ophthalmol. 160(6):1200–1210
Moein HR, Novais EA, Rebhun CB, Cole ED, Louzada RN, Witkin AJ et al (2017) Optical coherence tomography angiography to detect macular capillary ischemia in patients with inner retinal changes after resolved diabetic macular edema. Retina
Brown DM, Campochiaro PA, Bhisitkul RB, Ho AC, Gray S, Saroj N et al (2011) Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 118(8):1594–1602
Miwa Y, Muraoka Y, Osaka R, Ooto S, Murakami T, Suzuma K et al (2017) Ranibizumab for macular edema after branch retinal vein occlusion: one initial injection versus three monthly injections. Retina. 37(4):702–709
Suzuki M, Ozawa Y, Kubota S, Hirasawa M, Miyake S, Noda K et al (2011) Neuroprotective response after photodynamic therapy: role of vascular endothelial growth factor. J Neuroinflammation. 8:176
Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T et al (2008) Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS One. 3(11):e3554
Noma H, Mimura T, Eguchi S (2013) Association of inflammatory factors with macular edema in branch retinal vein occlusion. JAMA Ophthalmol. 131(2):160–165
Coscas F, Glacet-Bernard A, Miere A, Caillaux V, Uzzan J, Lupidi M et al (2016) Optical coherence tomography angiography in retinal vein occlusion: evaluation of superficial and deep capillary plexa. Am J Ophthalmol. 161(160-71):e1–e2
Kang JW, Yoo R, Jo YH, Kim HC (2017) Correlation of microvascular structures on optical coherence tomography angiography with visual acuity in retinal vein occlusion. Retina 37(9):1700–1709
Kanakis MG, Giannouli K, Andreanos K, Papaconstantinou D, Koutsandrea C, Ladas I et al (2017) Capillary nonperfusion and photoreceptor loss in branch retinal vein occlusion: spatial correlation and morphologic characteristics. Retina 37(9):1710–1722
Bakhoum MF, Freund KB, Dolz-Marco R, Leong BCS, Baumal CR, Duker JS et al (2018) Paracentral acute middle maculopathy and the ischemic cascade associated with retinal vascular occlusion. Am J Ophthalmol. 195:143–153
Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y et al (2017) Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. 7:42201
Acknowledgments
The authors thank all the clinical staff members for assistance at the Vitreo-Retina Surgical Division Clinic.
Data statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MS has received a speaker honorarium from Novartis Pharmaceuticals Japan. TK has received grants from Tsubota Laboratory, Inc., Fuji Xerox Co., Ltd., SEED Co., Ltd., Kowa Company, Santen Pharmaceutical Co. Ltd., and ROHTO Pharmaceutical Co., Ltd.; grants and speaker honorariums from Novartis Pharmaceuticals Japan; and personal fees from Bayer Yakuhin, Ltd.; all of these grants, honorariums, and fees were outside of the scope of the submitted work. KT reports research funding and consultancies from Santen Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co., Ltd., and Wakamoto Pharmaceutical Co., Ltd.; patents with JINS, Inc., Echo Electricity Co., and Kowa Company; KT is also the CEO of Tsubota Laboratory, Inc.; all of these grants, consultancies, patents, and employment arrangements were outside of the scope of the submitted work. YO received grants and speaker honorariums from Santen Pharmaceutical Co. Ltd., Novartis Pharmaceuticals Japan, Bayer Yakuhin, Ltd., Nitto Medic, Co. Ltd., Wakasa Seikatsu, Co. Ltd.; all of these grants and honorariums were outside of the scope of the submitted work. NN, SM, MK, HS, KW, and HS declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Keio University School of Medicine, Tokyo, Japan, and were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Table 1
Data at first remission of macular edema and at month 12 (DOCX 80 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Suzuki, M., Nagai, N., Minami, S. et al. Predicting recurrences of macular edema due to branch retinal vein occlusion during anti-vascular endothelial growth factor therapy. Graefes Arch Clin Exp Ophthalmol 258, 49–56 (2020). https://doi.org/10.1007/s00417-019-04495-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04495-9