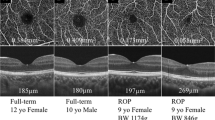
Abstract
Purpose
To assess foveal microvascular structure and the correlation between foveal retinal thickness and best corrected visual acuity (BCVA) in children with retinopathy of prematurity (ROP).
Methods
This is a retrospective case-control study. A total 42 eyes in 23 patients with history of anti-vascular endothelial factor (VEGF) agent treatment and 51 eyes of 27 healthy age-matched subjects as the control group were analyzed. Foveal avascular zone (FAZ) and foveal vessel density (VD) were measured by optical coherence tomography angiography (OCT-A). Foveal thickness was measured by cross-sectional OCT. Correlations between FAZ area, foveal VD, foveal thickness, BCVA, gestational age (GA), and birth body weight (BBW) were performed.
Results
ROP children had a significantly smaller FAZ area and higher foveal VD, and the foveal thickness was significantly higher as compared to controls (all P < 0.0001). We noted a significant negative correlation between FAZ area and foveal thickness. In addition, a significant positive correlation between foveal VD and foveal thickness was identified. With regard to prematurity status, gestational age and birth body weight were both significantly correlated with FAZ area, foveal VD, and fovea inner retinal thickness. Multivariable analysis showed that thicker inner retinal thickness and higher superficial vascular density were associated with suboptimal visual acuity.
Conclusion
By using OCT-A, we identified significant foveal microvascular anomalies in ROP children. The correlation between the microvascular anomalies, central foveal thickness, and suboptimal visual acuity was also noted. Because of the retrospective nature, more studies are necessary to further establish the relationship.

Similar content being viewed by others
References
Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362(6423):841–844
Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE (1995) Vascular endothelial growthfactor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A 92(3):905–909
Hartnett ME, Penn JS (2013) Mechanisms and management of retinopathy of prematurity. N Engl J Med 368(12):1162–1163
Mintz-Hittner HA, Prager TC, Kretzer FL (1992) Visual acuity correlates with severity ofretinopathy of prematurity in untreated infants weighing 750 g or less at birth. Arch Ophthalmol 110(8):1087–1091
Mintz-Hittner HA, Knight-Nanan DM, Satriano DR, Kretzer FL (1999) A small foveal avascular zone may be an historic mark of prematurity. Ophthalmology 106(7):1409–1413
Ecsedy M, Szamosi A, Karkó C, Zubovics L, Varsányi B, Németh J, Récsán Z (2007) A comparison of macular structure imaged by optical coherence tomography in preterm and full-term children. Invest Ophthalmol Vis Sci 48:5207–5211
Vinekar A, Avadhani K, Sivakumar M, Mahendradas P, Kurian M, Braganza S, Shetty R, Shetty BK (2011) Understanding clinically undetected macular changes in early retinopathy of prematurity on spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 52:5183–5188
Vinekar A, Mangalesh S, Jayadev C, Maldonado RS, Bauer N, Toth CA (2015) Retinal imaging of infants on spectral domain optical coherence tomography Biomed Res Int 782420
Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D (2012) Split-spectrum amplitude-decorrelation angiographywith optical coherence tomography. Opt Express 20(4):4710–4725
Spaide RF, Klancnik JM Jr, Cooney MJ (2015) Retinal vascular layers imaged byfluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 133(1):45–50
Campbell JP, Nudleman E, Yang J, Tan O, Chan RVP, Chiang MF, Huang D, Liu G (2017) Handheld optical coherence tomography angiography and ultra-wide-field optical coherence tomography in retinopathy of prematurity. JAMA Ophthalmol 135(9):977–981
Vinekar A, Chidambara L, Jayadev C, Sivakumar M, Webers CA, Shetty B (2016) Monitoring neovascularization in aggressive posterior retinopathy of prematurity using optical coherence tomography angiography. J AAPOS 20(3):271–274
Falavarjani KG, Iafe NA, Velez FG, Schwartz SD, Sadda SR, Sarraf D, Tsui I (2017) Optical coherence tomography angiography of the fovea in children born preterm. Retina 37(12):2289–2294
Nonobe N, Kaneko H,Ito Y,Takayama K,Kataoka K,Tsunekawa T,Matsuura T,Suzumura A,Shimizu H,Terasaki H (2017) Optical coherence tomographyangiography of the foveal avascular zone in children with a history of treatment-requiring retinopathy of prematurity. Retina DOI: https://doi.org/10.1097/IAE.0000000000001937
Kraus MF, Potsaid B, Mayer MA, Bock R, Baumann B, Liu JJ, Hornegger J, Fujimoto JG (2012) Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed Opt Express 3(6):1182–1199
Provis JM (2001) Development of the primate retinal vasculature. Prog Retin Eye Res 20(6):799–821
Engerman RL (1976) Development of the macular circulation. Invest Ophthalmol Vis Sci 15(10):835–840
Springer AD, Hendrickson AE (2005) Development of the primate area of high acuity, 3: temporal relationships between pit formation, retinal elongation and cone packing. Vis Neurosci 22(2):171–185
Böhm MR, Hodes F, Brockhaus K, Hummel S, Schlatt S, Melkonyan H, Thanos S (2016) Is Angiostatin involved in physiological foveal Avascularity? Invest Ophthalmol Vis Sci 57(11):4536–4552
Springer AD, Hendrickson AE (2004) Development of the primate area of high acuity. 1. Use of finite element analysis models to identify mechanical variables affecting pit formation. Vis Neurosci 21:53–62
Wu WC, Lin RI, Shih CP, Wang NK, Chen YP, Chao AN, Chen KJ, Chen TL, Hwang YS, Lai CC, Huang CY, Tsai S (2012) Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology 119(9):1907–1916
Gursoy H, Bilgec MD, Erol N, Basmak H, Colak E (2016) The macular findings on spectral-domain optical coherence tomography in premature infants with or without retinopathy of prematurity. Int Ophthalmol 36(4):591–600
Dubis AM, Subramaniam CD, Godara P, Carroll J, Costakos DM (2013) Subclinical macular findings in infants screened for retinopathy of prematurity with spectral-domain optical coherence tomography. Ophthalmology 120(8):1665–1671
Wang J, Spencer R, Leffler JN, Birch EE (2012) Critical period for foveal fine structure in children with regressed retinopathy of prematurity. Retina 32(2):330–339
Pueyo V, González I, Altemir I, Pérez T, Gómez G, Prieto E, Oros D (2015) Microstructural changes in the retina related to prematurity. Am J Ophthalmol 159(4):797–802
Hammer DX, Iftimia NV, Ferguson RD, Bigelow CE, Ustun TE, Barnaby AM, Fulton AB (2008) Foveal fine structure in retinopathy of prematurity: an adaptive optics Fourier domain optical coherence tomography study. Invest Ophthalmol Vis Sci 49(5):2061–2070
Yanni SE, Wang J, Chan M, Carroll J, Farsiu S, Leffler JN, Spencer R, Birch EE (2012) Foveal avascular zone and foveal pit formation after preterm birth. Br J Ophthalmol 96(7):961–966
Villegas VM, Capó H, Cavuoto K, McKeown CA, Berrocal AM (2014) Foveal structure-function correlation in children with history of retinopathy of prematurity. Am J Ophthalmol 158(3):508–512
Stoica F, Chirita-Emandi A, Andreescu N, Stanciu A, Zimbru CG, Puiu M (2017) Clinical relevance of retinal structure in children with laser-treated retinopathy of prematurity versus controls - using optical coherence tomography. Acta Ophthalmol. https://doi.org/10.1111/aos.13536
Fieß A, Janz J, Schuster AK, Kölb-Keerl R, Knuf M, Kirchhof B, Muether PS, Bauer J (2017) Macular morphology in former preterm and full-term infants aged 4 to 10 years. Graefes Arch Clin Exp Ophthalmol 255:1433–1442
Shao Z, Dorfman AL, Seshadri S (2011) Choroidal involution is a key component of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 52:6238–6248
Balasubramanian S, Borrelli E, Lonnigi M, Vele Z, Sarraf D, Sadda SR, Tsui I (2018) Visual function and optical coherence tomography angiography features in children born preterm. Retina. https://doi.org/10.1097/IAE.0000000000002301
Magrath GN, Say EAT, Sioufi K, Ferenczy S, Samara WA, Shields CL (2017) Variability in foveal avascular zone and capillary density using optical coherence tomography angiography machines in healthy eyes. Retina 37(11):2102–2111
Carpineto P, Mastropasqua R, Marchini G, Toto L, Di Nicola M, Di Antonio L (2016) Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol 100(5):671–676
Chiang MF, Jiang L, Gelman R, Du Y (2007) Inter expert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol 125:875–880
Wallace D, Quinn G, Freedman S, Chiang MF (2008) Agreement among pediatric ophthalmologists in diagnosing plus and pre-plus disease in retinopathy of prematurity. J AAPOS 12:352–356
Koreen S, Gelman R, Martinez-Perez ME, Jiang L, Berrocal AM, Hess DJ, Flynn JT, Chiang MF (2007) Evaluation of a computer-based system for plus disease diagnosis in retinopathy of prematurity. Ophthalmology 114:e59–e67
Campbell JP, Ryan MC, LoreE TP, Ostmo S, Jonas K, Chan RVP, Chiang M (2016) Diagnostic discrepancies in retinopathy of prematurity classification. Ophthalmology 123:1795–1801
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. The study was approved by the Institutional Review Board of the hospital and was conducted in accordance with the tenets of the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Chen, YC., Chen, YT. & Chen, SN. Foveal microvascular anomalies on optical coherence tomography angiography and the correlation with foveal thickness and visual acuity in retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 257, 23–30 (2019). https://doi.org/10.1007/s00417-018-4162-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-4162-y