Abstract
Purpose
This study aimed to analyse shifts in renin–angiotensin system (RAS) components, angiogenesis, and oxidative stress-related protein expression in the lamina cribrosa (LC) region in streptozotocin (STZ)-induced diabetic mice.
Methods
Six months after diabetes induction, the retinal vessels of male C57BL/6 J mice were observed by colour photography, fundus fluorescein angiography (FFA), and immunofluorescent staining following incubation with CD31. Immunofluorescence for glial fibrillary acidic protein (GFAP), alpha-smooth muscle actin (α-SMA),and NG2 was also performed. Angiotensin-converting enzyme 1 (ACE1), angiotensin II type I receptor (AT1R), renin, hypoxia-inducible factor 1-alpha (HIF-1α), vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor 2 (VEGFR2), and haeme oxygenase 1 (HO-1) expression levels were confirmed by immunohistochemical and western blotting analyses.
Results
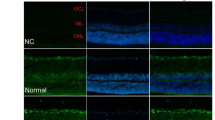
Compared with control mice, diabetic mice had significantly higher blood glucose concentrations (p < 0.001) and significantly lower body weights (p < 0.001). Colour photography and FFA did not reveal any vessel abnormalities in the diabetic mice; however, immunostaining of whole-mount retinas revealed an increased number of retinal vessels. Furthermore, histopathological staining showed significant reduction in the whole retinal thickness. GFAP expression was slightly higher, whereas fewer NG2+ pericytes were observed in diabetic mice than in control mice. ACE1, AT1R, renin, HIF-1α, VEGF, VEGFR2, and HO-1 expression were up-regulated in the LC of the STZ-induced diabetic mice.
Conclusions
Collectively, ACE 1, AT1R, HIF-1α, VEGF, VEGFR2, and HO-1 activation in the LC region in diabetic mice may be involved in diabetes via the RAS and induction of angiogenesis and oxidative stress.







Similar content being viewed by others
References
Li WL, Zheng HC, Bukuru J, De Kimpe N (2004) Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol 92:1–21. https://doi.org/10.1016/j.jep.2003.12.031
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Simo R, Hernandez C (2012) Prevention and treatment of diabetic retinopathy: evidence from large, randomized trials. The emerging role of fenofibrate. Rev Recent Clin Trials 7:71–80
Bandello F, Lattanzio R, Zucchiatti I, Del TC (2013) Pathophysiology and treatment of diabetic retinopathy. Acta Diabetol 50:1–20. https://doi.org/10.1007/s00592-012-0449-3
Kollias AN, Ulbig MW (2010) Diabetic retinopathy: early diagnosis and effective treatment. Dtsch Arztebl Int 107(75–83):84. https://doi.org/10.3238/arztebl.2010.0075
Funk RH (1997) Blood supply of the retina. Ophthalmic Res 29:320–325
Kang MH, Balaratnasingam C, Yu PK, Morgan WH, McAllister IL, Cringle SJ, Yu DY (2011) Morphometric characteristics of central retinal artery and vein endothelium in the normal human optic nerve head. Invest Ophthalmol Vis Sci 52:1359–1367. https://doi.org/10.1167/iovs.10-6366
Anderson DR (1969) Ultrastructure of human and monkey lamina cribrosa and optic nerve head. Arch Ophthalmol 82:800–814
Hernandez MR, Igoe F, Neufeld AH (1988) Cell culture of the human lamina cribrosa. Invest Ophthalmol Vis Sci 29:78–89
Tovar-Vidales T, Wordinger RJ, Clark AF (2016) Identification and localization of lamina cribrosa cells in the human optic nerve head. Exp Eye Res 147:94–97. https://doi.org/10.1016/j.exer.2016.05.006
Balaratnasingam C, Kang MH, Yu P, Chan G, Morgan WH, Cringle SJ, Yu DY (2014) Comparative quantitative study of astrocytes and capillary distribution in optic nerve laminar regions. Exp Eye Res 121:11–22. https://doi.org/10.1016/j.exer.2014.02.008
Williamson TH (2007) A “throttle” mechanism in the central retinal vein in the region of the lamina cribrosa. Br J Ophthalmol 91:1190–1193. https://doi.org/10.1136/bjo.2006.102798
Dong Y, Lin L, Yan H, Fu Y, Zong Y, Yuan Y, Huang X, Li Y, He H, Gao Q (2016) Shifts in retinal vessel diameter and oxygen saturation in Chinese type 2 diabetes mellitus patients. BMC Ophthalmol 16:43. https://doi.org/10.1186/s12886-016-0217-1
Gurley SB, Coffman TM (2007) The renin-angiotensin system and diabetic nephropathy. Semin Nephrol 27:144–152. https://doi.org/10.1016/j.semnephrol.2007.01.009
Agarwal P, Jindal A, Saini VK, Jindal S (2014) Advances in diabetic retinopathy. Indian J Endocrinol Metab 18:772–777. https://doi.org/10.4103/2230-8210.140225
Campochiaro PA (2015) Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res 49:67–81. https://doi.org/10.1016/j.preteyeres.2015.06.002
Wan TT, Li XF, Sun YM, Li YB, Su Y (2015) Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. Biomed Pharmacother 74:145–147. https://doi.org/10.1016/j.biopha.2015.08.002
Moravski CJ, Skinner SL, Stubbs AJ, Sarlos S, Kelly DJ, Cooper ME, Gilbert RE, Wilkinson-Berka JL (2003) The renin-angiotensin system influences ocular endothelial cell proliferation in diabetes: transgenic and interventional studies. Am J Pathol 162:151–160. https://doi.org/10.1016/S0002-9440(10)63806-0
Fukuda A, Wickman LT, Venkatareddy MP, Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE, Wiggins RC (2012) Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int 81:40–55. https://doi.org/10.1038/ki.2011.306
Ola MS, Alhomida AS, Ferrario CM, Ahmad S (2017) Role of tissue Renin-angiotensin system and the Chymase/angiotensin-(1-12) axis in the pathogenesis of diabetic retinopathy. Curr Med Chem. https://doi.org/10.2174/0929867324666170407141955
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25:581–611. https://doi.org/10.1210/er.2003-0027
Schrufer TL, Antonetti DA, Sonenberg N, Kimball SR, Gardner TW, Jefferson LS (2010) Ablation of 4E-BP1/2 prevents hyperglycemia-mediated induction of VEGF expression in the rodent retina and in Muller cells in culture. Diabetes 59:2107–2116. https://doi.org/10.2337/db10-0148
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Et A (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487. https://doi.org/10.1056/NEJM199412013312203
Ferrara N (2005) The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS:209–231
Brozzo MS, Bjelic S, Kisko K, Schleier T, Leppanen VM, Alitalo K, Winkler FK, Ballmer-Hofer K (2012) Thermodynamic and structural description of allosterically regulated VEGFR-2 dimerization. Blood 119:1781–1788. https://doi.org/10.1182/blood-2011-11-390922
Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH (2000) Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem 275:25733–25741. https://doi.org/10.1074/jbc.M002740200
Ling S, Birnbaum Y, Nanhwan MK, Thomas B, Bajaj M, Ye Y (2013) MicroRNA-dependent cross-talk between VEGF and HIF1alpha in the diabetic retina. Cell Signal 25:2840–2847. https://doi.org/10.1016/j.cellsig.2013.08.039
Yan HT, Su GF (2014) Expression and significance of HIF-1 alpha and VEGF in rats with diabetic retinopathy. Asian Pac J Trop Med 7:237–240. https://doi.org/10.1016/S1995-7645(14)60028-6
Zhang M, Gao X, Bai SJ, Ye XM, Zhang J (2014) Effect of pioglitazone on expression of hypoxia-inducible factor 1alpha and vascular endothelial growth factor in ischemic hindlimb of diabetic rats. Eur Rev Med Pharmacol Sci 18:1307–1314
Berkowitz BA, Grady EM, Khetarpal N, Patel A, Roberts R (2015) Oxidative stress and light-evoked responses of the posterior segment in a mouse model of diabetic retinopathy. Invest Ophthalmol Vis Sci 56:606–615. https://doi.org/10.1167/iovs.14-15687
Sharma S, Saxena S, Srivastav K, Shukla RK, Mishra N, Meyer CH, Kruzliak P, Khanna VK (2015) Nitric oxide and oxidative stress is associated with severity of diabetic retinopathy and retinal structural alterations. Clin Experiment Ophthalmol 43:429–436. https://doi.org/10.1111/ceo.12506
Lamoke F, Shaw S, Yuan J, Ananth S, Duncan M, Martin P, Bartoli M (2015) Increased oxidative and Nitrative stress accelerates aging of the retinal vasculature in the diabetic retina. PLoS One 10:e139664. https://doi.org/10.1371/journal.pone.0139664
Castilho A, Aveleira CA, Leal EC, Simoes NF, Fernandes CR, Meirinhos RI, Baptista FI, Ambrosio AF (2012) Heme oxygenase-1 protects retinal endothelial cells against high glucose- and oxidative/nitrosative stress-induced toxicity. PLoS One 7:e42428. https://doi.org/10.1371/journal.pone.0042428
Son E, Jeong J, Lee J, Jung DY, Cho GJ, Choi WS, Lee MS, Kim SH, Kim IK, Suk K (2005) Sequential induction of heme oxygenase-1 and manganese superoxide dismutase protects cultured astrocytes against nitric oxide. Biochem Pharmacol 70:590–597. https://doi.org/10.1016/j.bcp.2005.05.027
Song Y, Huang L, Yu J (2016) Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf2/HO-1 signaling. J Neuroimmunol 301:1–6. https://doi.org/10.1016/j.jneuroim.2016.11.001
Ma C, Long H (2016) Protective effect of betulin on cognitive decline in streptozotocin (STZ)-induced diabetic rats. Neurotoxicology 57:104–111. https://doi.org/10.1016/j.neuro.2016.09.009
Hernandez MR (2000) The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res 19:297–321
Wallace DM, O'Brien CJ (2016) The role of lamina cribrosa cells in optic nerve head fibrosis in glaucoma. Exp Eye Res 142:102–109. https://doi.org/10.1016/j.exer.2014.12.006
Schneider M, Fuchshofer R (2016) The role of astrocytes in optic nerve head fibrosis in glaucoma. Exp Eye Res 142:49–55. https://doi.org/10.1016/j.exer.2015.08.014
Rosenstein JM, Krum JM, Ruhrberg C (2010) VEGF in the nervous system. Organogenesis 6:107–114
Crawford DJ, Roberts MD, Sigal IA (2011) Glaucomatous cupping of the lamina cribrosa: a review of the evidence for active progressive remodeling as a mechanism. Exp Eye Res 93:133–140. https://doi.org/10.1016/j.exer.2010.08.004
Gong CY, Yu ZY, Lu B, Yang L, Sheng YC, Fan YM, Ji LL, Wang ZT (2014) Ethanol extract of Dendrobium chrysotoxum Lindl ameliorates diabetic retinopathy and its mechanism. Vasc Pharmacol 62:134–142. https://doi.org/10.1016/j.vph.2014.04.007
Kriechbaum K, Prager S, Mylonas G, Scholda C, Rainer G, Funk M, Kundi M, Schmidt-Erfurth U (2014) Intravitreal bevacizumab (Avastin) versus triamcinolone (Volon a) for treatment of diabetic macular edema: one-year results. Eye (Lond) 28:9–15. 16. https://doi.org/10.1038/eye.2013.242
Ip MS, Domalpally A, Sun JK, Ehrlich JS (2015) Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology 122:367–374. https://doi.org/10.1016/j.ophtha.2014.08.048
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307. https://doi.org/10.1038/nature10144
Butt AM, Pugh M, Hubbard P, James G (2004) Functions of optic nerve glia: axoglial signalling in physiology and pathology. Eye (Lond) 18:1110–1121. https://doi.org/10.1038/sj.eye.6701595
HOGAN MJ, FEENEY L (1963) The ULTRASTRUCTURE of the retinal blood vessels. I. The large vessels. J Ultrastruct Res 39:10–28
Ransom BR, Orkand RK (1996) Glial-neuronal interactions in non-synaptic areas of the brain: studies in the optic nerve. Trends Neurosci 19:352–358
Downs JC, Girkin CA (2017) Lamina cribrosa in glaucoma. Curr Opin Ophthalmol 28:113–119. https://doi.org/10.1097/ICU.0000000000000354
Morrison JC (2006) Integrins in the optic nerve head: potential roles in glaucomatous optic neuropathy (an American ophthalmological society thesis). Trans Am Ophthalmol Soc 104:453–477
Madsen-Bouterse SA, Kowluru RA (2008) Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord 9:315–327. https://doi.org/10.1007/s11154-008-9090-4
Payne AJ, Kaja S, Naumchuk Y, Kunjukunju N, Koulen P (2014) Antioxidant drug therapy approaches for Neuroprotection in chronic diseases of the retina. Int J Mol Sci 15:1865–1886. https://doi.org/10.3390/ijms15021865
Amano S, Kaji Y, Oshika T, Oka T, Machinami R, Nagai R, Horiuchi S (2001) Advanced glycation end products in human optic nerve head. Br J Ophthalmol 85:52–55
Kern TS, Engerman RL (1994) Comparison of retinal lesions in alloxan-diabetic rats and galactose-fed rats. Curr Eye Res 13:863–867
Huang SS, Khosrof SA, Koletsky RJ, Benetz BA, Ernsberger P (1995) Characterization of retinal vascular abnormalities in lean and obese spontaneously hypertensive rats. Clin Exp Pharmacol Physiol Suppl 22:S129–S131
Acknowledgements
We thank Zhiquan Li taking care of mice, Xiaoxiao Feng and Cheng Li, for developmental review of the manuscript.
Funding
This study was funded by the National Science & Technology Pillar Program of the Twelfth Five-year Plan (2012BAI08B00), the Science and Technology Planning Project of Guangdong Province, China (2015B020211004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Qian, X., Lin, L., Zong, Y. et al. Shifts in renin–angiotensin system components, angiogenesis, and oxidative stress-related protein expression in the lamina cribrosa region of streptozotocin-induced diabetic mice. Graefes Arch Clin Exp Ophthalmol 256, 525–534 (2018). https://doi.org/10.1007/s00417-017-3866-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3866-8