Abstract
Purpose
To test the hypothesis of a significant relationship between systemic markers of renal and vascular function (processes linked to cardiovascular disease and its development) and retinal microvascular function in diabetes and/or cardiovascular disease.
Methods
Ocular microcirculatory function was measured in 116 patients with diabetes and/or cardiovascular disease using static and continuous retinal vessel responses to three cycles of flickering light. Endothelial function was evaluated by von Willebrand factor (vWf), endothelial microparticles and soluble E selectin, renal function by serum creatinine, creatinine clearance and estimated glomerular filtration rate (eGFR). HbA1c was used as a control index.
Results
Central retinal vein equivalence and venous maximum dilation to flicker were linked to HbA1c (both p < 0.05). Arterial reaction time was linked to serum creatinine (p = 0.036) and eGFR (p = 0.039); venous reaction time was linked to creatinine clearance (p = 0.018). Creatinine clearance and eGFR were linked to arterial maximum dilatation (p < 0.001 and p = 0.003, respectively) and the dilatation amplitude (p = 0.038 and p = 0.048, respectively) responses in the third flicker cycle. Of venous responses to the first flicker cycle, HbA1c was linked to the maximum dilation response (p = 0.004) and dilatation amplitude (p = 0.017), vWf was linked to the maximum constriction response (p = 0.016), and creatinine clearance to the baseline diameter fluctuation (p = 0.029). In the second flicker cycle, dilatation amplitude was linked to serum creatinine (p = 0.022).
Conclusions
Several retinal blood vessel responses to flickering light are linked to glycaemia and renal function, but only one index is linked to endothelial function. Renal function must be considered when interpreting retinal vessel responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The relatively straightforward non-invasive assessment of the retinal circulation by methods such as fundus photography, video recording and tomography has led to the view that it has potential as a screening tool for cardiovascular disease such as myocardial infarction and stroke [1–3]. This concept has been supported through large population studies [4–6] that together demonstrate a relationship between certain retinal vessel indices and cardiovascular risk [7, 8]. Individuals with good cardiovascular health are less likely to have signs of retinopathy such as dilated retinal venules and narrow retinal arterioles, both of which are associated with increased risk of stroke and coronary artery disease [9].
Whilst retinal vessel calibres provide only static indices, dynamic measurements such as retinal vessel reactivity to flicker light provocation can provide further insight into the status of the retinal microcirculation. Several authors have demonstrated a link between measures of cardiovascular health and retinal vessel dynamics, such as a decrease in retinal vessel dilation in the presence of decreased flow mediated dilation of the brachial artery [10], prolonged reaction times in retinal arterial responses to flicker light in patients suffering from coronary artery disease [11], and decreased vessel dilation to flicker light in patients with coronary artery disease, and which depends on the severity of the disease [12]. There is considerable evidence that retinal vessel reactivity to flicker light provocation is blunted in the presence of diabetes and in diabetic retinopathy [13–16].
The classical risk factors for the development of cardiovascular disease operate at the level of the endothelium [17, 18]. Leading plasma markers of endothelial perturbation, known to be abnormal in cardiovascular disease, include von Willebrand factor, soluble E selection and endothelial microparticles [19–21]. A further complicating pathogenic process in cardiovascular disease is poor renal function, known to be present in hypertension and diabetes [22–24], and which is a risk factor for major cardiovascular disease [25, 26]. We therefore hypothesised that retinal arterial and venous static and flicker responses are influenced by renal and/or vascular function. We tested our hypothesis in a clinically relevant cohort of patients with diabetes and/or cardiovascular disease.
Materials and methods
Subjects
We recruited 116 patients from out-patient clinics at a University Teaching Hospital. Inclusion criteria were history of cardiovascular disease (myocardial infarction, stroke, >50 % stenosis of artery proven by transcutaneous intervention, artery bypass grafting, amputation) and diabetes (HbA1c > 55 mmol/mol and/or attendance at a diabetes clinic). Exclusion criteria were age <18 years, connective tissue disease, cancer, recent (<3 months) cardiovascular events such as myocardial infarction or stroke, recent (<3 months) surgery, established ocular disease such as age-related macular degeneration. Ethical approval was obtained from West Birmingham Ethics Committee and Aston University Ethics Committee. Written informed consent was received from all individuals taking part in the study. This study has been designed and conducted in accordance with the Declaration of Helsinki.
Study protocol
A full history and examination took place to ensure that subjects were free from any disease as outlined in the exclusion criteria. All subjects were instructed to refrain from consuming caffeinated products, chocolate, drinking alcohol and smoking on the study day. Intraocular pressure (IOP) was measured by contact tonometry and calculated as the mean of three consecutive readings after instillation of one drop of 0.4 % benoxinate hydrochloride (Chauvin Pharmaceuticals Ltd., Kingston-Upon-Thames, UK; TonopenXL, Medtronic Solan, PMS Instruments, Maidenhead, UK). Data were discarded if the coefficient of variation (CV) exceeded 5 %.
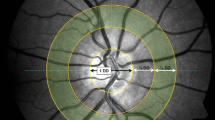
Dynamic and static retinal vessel assessment was determined after full pupil dilation was reached with 1 % tropicamide (Chauvin Pharmaceuticals Ltd., Kingston-Upon-Thames, UK), digital fundus images and reactivity parameters of retinal blood arteries and veins were obtained (retinal vessel analyser [RVA], Imedos Systems (UG) haftungsbeschraenkt Jena, Germany) [27]. For static vessel analysis, black and white fundus images were obtained at a 30° angle with the optic nerve head centred using the inbuilt Zeiss 450 F fundus camera (Zeiss GmbH, Germany). Arterial and venous diameters provided an arteriovenous ratio (AVR), central retinal artery equivalent (CRAE) and central retinal vein equivalent (CRVE; Vesselmap software, Imedos Systems (UG) haftungsbeschraenkt, Jena, Germany) [28, 29]. CRVE, CRAE and AVR were calculated from arteries and veins that were located within a ring whose centre was the optic nerve head and whose inner and outer margins were of one half disc diameter (DD) and one full DD. These measurements (AVR, CRAE and CRVE) are standard ophthalmological indices and are used to describe the physical structural of different retinal arteries and veins such as luminal diameter.
Static imaging was followed by dynamic assessment where retinal diameters were measured continuously at a sampling rate of 25 Hz. Stimulation of retinal blood vessels was done by optoelectronic interruption of the green fundus illumination used by the RVA resulting in a flickering light provocation with a 12.5-Hz frequency [30–32]. After BP stabilisation and image focussing, a vessel segment of the superior temporal retinal artery and vein (500 μm in length) was chosen at a distance of 1.5–2 DD away from the margins of the optic nerve head. Baseline diameter of both the artery and vein was recorded according to the standard RVA protocol [32] for 50 s and then followed by 3 cycles of 20-s flicker provocation with each 80-s recovery time. This resulted in a 350-s measuring period during which the fellow eye was occluded. From the diameter recordings, the values for maximum dilation (MD), maximum constriction (MC) and dilation amplitude (DA), arterial baseline corrected flicker response (BFR), and arterial and venous reaction time (RT) to flicker provocation were calculated [33].
Plasma markers
Venous blood was collected into citric acid and plasma obtained after centrifugation at 1000 g for 20 min. Von Willebrand factor (vWf) and soluble E selectin were measured by commercial enzyme linked immune-sorbent assay (ELISA; Dako-Cytomation, Ely, Cambs UK and R&D Systems, Abingdon, UK). The ELISAs had intra- and inter-assay coefficients of variation <5 % and <10 %, respectively. Endothelial microparticles were determined by flow cytometry (Apogee Flow Systems, Hemel Hemstead, UK) using fluorochrome-linked monoclonal antibodies to CD144 (R&D Systems, Abingdon, UK) [34]. The size of the EMP was confirmed with polystyrene beads of 110-, 200-, 500-nm and 1-μm diameter together with 300- and 880-nm silica beads (Apogee Flow Systems, Hemel Hemstead, UK). Creatinine and HbA1c were measured by standard techniques by the Hospital Routine Pathology Laboratory. Creatinine clearance and the estimated glomerular filtration rate (eGFR) were calculated according to the Cockcroft and Gault, and the Modification of Diet in Renal Disease equations, respectively [35, 36].
Statistics
We hypothesised that renal function (as defined by creatinine clearance, serum creatinine and eGFR), systemic endothelial function (as marked by endothelial microparticles, von Willebrand factor and soluble E selectin) and glycaemia (as assessed by HbA1c) each have an effect on retinal vessel function. We tested these hypotheses in a multi-variate linear regression analysis taking each ocular index and the dependent variable and the seven research indices as independent variables. According to Altman [37], a sample size of at least ten is required for each independent variable, of which we have seven, thus calling for a total sample size of at least 70 patients. However, in view of the possible likelihood of a relationship between the seven indices, and in order to obtain greater confidence we decided to over-recruit by at least 50 % (i.e. to at least 105 patients), eventually recruiting 116 patients. Continuously variable data are presented as mean and standard deviation or as median and interquartile range as distribution demands, and were correlated by Spearman’s method. Categorical data are presented as number and percentage. Analyses were performed on Minitab version 17 (Minitab Inc, Coventry, UK).
Results
Tables 1, 2 and 3 show the clinical, demographic and ocular indices of all 116 patients. Median duration of disease in the 73 diabetics was 10 years (interquartile range 4.5–16.5 years).
In the analyses of the ocular indices of Table 3(a), although intraocular pressure was linked to creatinine clearance (p = 0.014), eGFR (p = 0.004) and serum creatinine (p = 0.011) in univariate analysis, none remained significant in multivariate analysis. HbA1c was linked to CRVE (p = 0.014) and venous size (p = 0.034), but there were no other links with other venous indices or any arterial index. In analysis of the IMEDOS indices, no laboratory or clinical index was significantly linked to any arterial index, but V max was linked to HbA1c (p = 0.038). Regarding the ocular indices in Table 3(b), no laboratory or clinical index was linked to averaged arterial maximum dilatation, constriction or dilatation amplitude or baseline diameter fluctuation, but in univariate analysis, the arterial reaction time was linked to serum creatinine (p = 0.033) and the eGFR (p = 0.035), and both were retained in multivariate analysis (p = 0.036 and p = 0.039, respectively). Of the averaged venous indices, once more, maximum dilatation, constriction or dilatation amplitude or baseline diameter fluctuation failed to link to any index. However, venous reaction time was linked to creatinine clearance (univariate p = 0.024, multivariate p = 0.018) and eGFR (univariate p = 0.033 but multivariate p = 0.126).
Correlations between the laboratory indices are present in Table 4. Von Willebrand factor correlated with soluble E selectin, whilst (unsurprisingly) the three renal indices strongly inter-correlated. For each ocular index, we first performed a univariate analysis of all seven research indices, then a multivariate analysis with only those indices that were significant (p < 0.05) in the univariate analysis. Analyses of the arterial and vein responses to the three individual flicker cycles in relation to the laboratory and haemodynamic indices are presented in Table 5. The creatinine clearance and eGFR were both independently linked to the arterial maximum dilatation response and the dilatation amplitude response to the third flicker cycle. As regards venous responses, in the first flicker cycle, HbA1c was linked to the venous maximum dilation response and dilatation amplitude, von Willebrand factor was linked to maximum constriction, and creatinine clearance was linked to baseline diameter fluctuation.
Discussion
The eye represents a unique opportunity to non-invasively assess microcirculation, and may be useful in predicting those at risk of cardiovascular disease [2–10, 38–40]. A further development in ocular pathology is the recognition of the value of retinal vessel responses to flickering light in diabetes and cardiovascular disease [14–16, 41–43]. A potential pathophysiological process to explain these abnormal retinal vessel responses is endothelial dysfunction [11–13, 17, 18, 44–46], known to be present in diabetes [47–49]. However, the kidney can also be a target organ in diabetes, and may also be linked to endothelial dysfunction [50–52]. Despite this, the role of renal function in retinal vessel responses to flicker light is unexplored.
We tested the hypothesis that retinal vessel responses in patients with diabetes and/or cardiovascular disease would be linked to vascular and/or renal function, but factored in glycaemia as a reference pathological process. As markers of endothelial function, we chose endothelial microparticles, von Willebrand factor and soluble E selectin [19, 20]. The former are shed from the cell membrane as a result of endothelial injury and in vasomotion disorders involved in cardiovascular disease, including diabetes [21, 53, 54]. Von Willebrand factor, a large multimeric glycoprotein stored in endothelial Weibel–Palade bodies and released in a steady state directly from translation and transcription, promotes platelet–platelet and platelet–subendothelial adhesion, and is a co-factor for coagulation factor VIII [55]. However, at times of increased endothelial stress, damage or activation, Wiebel–Palade bodies release large amounts into the plasma, thereby promoting thrombosis [56]. Increased levels in diabetes and diabetic retinopathy are a long-established fact [57, 58]. The adhesion molecule E-selectin (CD69E) is a membrane component upregulated at times of endothelial activation, under which conditions it mediates adhesion between the endothelium and leukocytes [59]. Serum proteases can generate a cleaved product, i.e. soluble E selectin, increased levels of which reflect increased endothelial activation [60, 61]. Potential mechanisms for increased plasma levels of these markers in diabetes are diverse and include the effects of hyperglyaemia, advanced glycation end products, reactive oxygen species and inflammation [49, 62, 63].
We found that HbA1c is linked to several venous retinal vessel indices — the CRVE, venous size, V max, and the maximum dilatation and dilatation amplitude to the first flicker cycle. No arterial index was linked to HbA1c. Only one vascular marker (von Willebrand factor, known to be increased in diabetes [57, 58, 64]) was linked to a retinal vessel response — that of venous maximum constriction. However, renal markers were linked to retinal vessel responses on several occasions. The arterial reaction time was linked to serum creatinine and the eGFR, and in the third flicker cycle, maximum dilation was linked to creatinine clearance and the eGFR, and dilation amplitude to creatinine clearance and the eGFR. However, venous reaction time was linked to creatinine clearance, and venous baseline diameter fluctuation was linked to creatinine clearance in the third flicker cycle.
Notably, no renal or vascular index was linked to intraocular pressure or to any of the IMEDOS indices, but several indices were linked to flicker response indices. The fact that arterial responses were linked to renal indices in the third, but not the first or second flicker cycles, is curious. This result, remaining present after adjustment for multiple analyses and factors, is robust and presumably reflects a pathophysiological feature of the arterial vessel that is apparent only after serial stimulation. This is counter to the link between vascular, renal and glycaemic indices and venous responses that were evident only in the first flicker cycle, when vessels were relatively unstimulated. We also note that soluble E selectin and endothelial microparticles, both known to be increased in diabetes [47, 53, 54, 65], were not linked to any ocular or retinal vessel index.
The most powerful relationships we found were between the positive correlations between two renal indices and arterial maximum dilation in the third flicker cycle. This we interpret as high maximum arterial dilations, reflecting good microvascular responses, are linked to good renal function as are reflected by high eGFR and high creatinine clearance. This is similar to the link between dilation amplitude and renal indices. The reason why these are present not in the first or second, but only in the third cycle may be that the vessels are less able to control their responses after a prolonged bout of stimulation.
The link between venous responses and HbA1c in the first flicker cycle alone is perhaps unsurprising given the weight of literature on the effect of this risk factor on the vasculature [39–44]. However, this is in contrast to the lack of an arterial link which supports the work of Mandecka et al. [14] who also failed to correlate HbA1c with flicker responses. The links with von Willebrand factor and creatinine clearance, although present and adjusted for multiple analyses, are, nonetheless, weak and so may be spurious.
Our data confirms, contrasts and extends that of others. Yip et al. [66] reported that retinopathy was associated with end-stage renal disease, the latter being unrelated to retinal arteriolar calibre, retinal venular calibre and retinal vascular fractal dimension. Eriksen et al. [67] found that the measured GFR, but not a creatinine-based eGFR, was linked to retinopathy, but not to retinal artery or vein diameters. Our data supports this finding as our creatinine-based eGFR did not correlate significantly with retinal artery or vein diameters. Using retinal fundus photography, Lim et al. [68] described an association between narrower retinal arteriolar calibre, smaller retinal vascular fractal dimensions and the presence of AV nicking and opacification with lower eGFR and a higher urinary albumin to creatinine ratio and microalbuminuria, the latter reflecting more severe renal disease. They concluded that quantitative changes of the retinal vascular geometry and qualitative changes in the vessel architecture are associated with markers of renal dysfunction and damage. Together, these three sets of data all support, to one extent or another, the general hypothesis that renal function is important in retinal vessel integrity, as does our own data.
We acknowledge the limitation of multiple analyses, and that many of these are likely to be physiologically (the vessel indices) and/or mathematically (the renal indices) related (correlations in Table 4) and so we may be at risk of false positives. However, we feel this is countered by the large sample size and that we have not over-interpreted our data. Our data may also be limited by possible effects of various systemic medications being taken by the patients, and that we recruited from a well-motivated group that may contribute to a better than expected endothelial function compared to poorly motivated patients on different medications. Nevertheless, our population is fully clinical in that all were being seen in a secondary care setting for diabetes and/or cardiovascular disease, and so represent the full spectrum of diabetic atherosclerosis.
We conclude that HbA1c may be an influence on venous responses to a first flicker stimulation, and that renal function is an influence on arterial responses to a third flicker cycle. Flicker stimulation analyses should consider the effects of these risk factors.
References
Sayin N, Kara N, Pekel G (2015) Ocular complications of diabetes mellitus. World J Diab 15:92–108
Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B (2005) Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat 206:319–348
Liew G, Wang JJ, Mitchell P, Wong TY (2008) Retinal vascular imaging: a new tool in microvascular disease research. Circ Cardiovasc Imaging 1:156–161
Flammer J, Konieczka K, Bruno RM, Virdis A, Flammer AJ, Taddei S (2013) The eye and the heart. Eur Heart J 34:1270–1278
Kawasaki R, Cheung N, Mosley T et al (2010) Retinal microvascular signs and 10-year risk of cerebral atrophy: the Atherosclerosis Risk in Communities (ARIC) study. Stroke 41:1826–1828
Kawasaki R, Xie J, Cheung N, Lamoureux E, Klein R, Klein BE et al (2012) Retinal microvascular signs and risk of stroke: the Multi-Ethnic Study of Atherosclerosis (MESA). Stroke 43:3245–3251
Liew G, Wang JJ, Rochtchina E, Wong TY, Mitchell P (2014) Complete blood count and retinal vessel calibers. PLoS One 18(9):e102230
Wang JJ, Liew G, Wong TY, Smith W, Klein R, Leeder SR et al (2006) Retinal vascular calibre and the risk of coronary heart disease-related death. Heart 92:1583–1587
von Hanno T, Bertelsen G, Sjølie AK, Mathiesen EB (2014) Retinal vascular calibres are significantly associated with cardiovascular risk factors: the Tromsø Eye Study. Acta Ophthalmol 92:40–46
Ogagarue ER, Lutsey PL, Klein R, Klein BE, Folsom AR (2013) Association of ideal cardiovascular health metrics and retinal microvascular findings: the Atherosclerosis Risk in Communities Study. J Am Heart Assoc 19(2):e000430
Pemp B, Weigert G, Karl K, Petzl U, Wolzt M, Schmetterer L et al (2009) Correlation of flicker-induced and flow-mediated vasodilatation in patients with endothelial dysfunction and healthy volunteers. Diabetes Care 32:1536–1541
Heitmar R, Cubbidge RP, Lip GY, Gherghel D, Blann AD (2011) Altered blood vessel responses in the eye and finger in coronary artery disease. Invest Ophthalmol Vis Sci 52:6199–6205
Al-Fiadh AH, Wong TY, Kawasaki R, Clark DJ, Patel SK, Freeman M et al (2015) Usefulness of retinal microvascular endothelial dysfunction as a predictor of coronary artery disease. Am J Cardiol 115:609–613
Mandecka A, Dawczynski J, Blum M, Müller N, Kloos C, Wolf G et al (2007) Influence of flickering light on the retinal vessels in diabetic patients. Diabetes Care 30:3048–3052
Nguyen TT, Kawasaki R, Kreis AJ, Wang JJ, Shaw J, Vilser W et al (2009) Correlation of light-flicker-induced retinal vasodilation and retinal vascular caliber measurements in diabetes. Invest Ophthalmol Vis Sci 50:5609–5613
Lim LS, Ling LH, Ong PG, Foulds W, Tai ES, Wong E (2014) Dynamic responses in retinal vessel caliber with flicker light stimulation in eyes with diabetic retinopathy. Invest Ophthalmol Vis Sci 55:5207–5213
Fischer-Rasokat U, Spyridopoulos I, Walter J, Honold J, Zeiher AM, Fichtlscherer S (2012) Microvascular dysfunction and pulse wave reflection characterize different vascular pathologies in patients at cardiovascular risk. Vasa 41:192–199
Madonna R, De Caterina R (2011) Cellular and molecular mechanisms of vascular injury in diabetes–part I: pathways of vascular disease in diabetes. Vascul Pharmacol 54:68–74
Blann AD, Taberner DA (1995) A reliable marker of endothelial cell dysfunction: does it exist? Br J Haematol 90:244–248
Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J et al (2011) Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil 18:775–789
Berezin A, Zulli A, Kerrigan S, Petrovic D, Kruzliak P (2015) Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clin Biochem 48:562–568
James MT, Grams ME, Woodward M, Elley CR, Green JA (2015) A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis 66:602–612
Garimella PS, Hirsch AT (2014) Peripheral artery disease and chronic kidney disease: clinical synergy to improve outcomes. Adv Chronic Kidney Dis 21:460–471
Di Lullo L, House A, Gorini A, Santoboni A, Russo D, Ronco C (2015) Chronic kidney disease and cardiovascular complications. Heart Fail Rev 20:259–272
Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP et al (2002) Cardiovascular disease risk status in elderly persons with renal insufficiency. Kidney Int 62:997–1004
El Husseini N, Kaskar O, Goldstein LB (2014) Chronic kidney disease and stroke. Adv Chronic Kidney Dis 21:500–508
Polak K, Dorner G, Kiss B, Polska E, Findl O, Rainer G et al (2000) Evaluation of the Zeiss retinal vessel analyser. Br J Ophthalmol 84:1285–1290
Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R et al (1999) Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the atherosclerosis risk in communities study. Ophthalmology 106:2269–2280
Parr JC, Spears GF (1974) General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. Am J Ophthalmol 77:472–477
Blum M, Bachmann K, Wintzer D, Riemer T, Vilser W, Strobel J (1999) Noninvasive measurement of the Bayliss effect in retinal autoregulation. Graefes Arch Clin Exp Ophthalmol 237:296–300
Seifertl BU, Vilser W (2002) Retinal Vessel Analyzer (RVA)–design and function. Biomed Tech 47(Suppl 1 Pt 2):678–681
Garhofer G, Bek T, Boehm AG, Gherghel D, Grunwald J, Jeppesen P et al (2010) Use of the retinal vessel analyzer in ocular blood flow research. Acta Ophthalmol 88:717–722
Heitmar R, Blann AD, Cubbidge RP, Lip GYH, Gherghel D (2010) Continuous retinal vessel diameter measurements - the future of retinal vessel assessment? Invest Ophthalmol Vis Sci 51:5833–5839
Montoro-García S, Shantsila E, Tapp LD, López-Cuenca A, Romero AI, Hernández-Romero D et al (2013) Small-size circulating microparticles in acute coronary syndromes: relevance to fibrinolytic status, reparative markers and outcomes. Atherosclerosis 227:313–322
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470
Altman DG (1991) Practical statistics for medical research. Chapman and Hall, London
Wong TY, Klein R, Klein BE, Tielsch JM, Hubbard L, Nieto FJ (2001) Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol 46:59–80
Fujioka S, Karashima K, Nishikawa N, Saito Y (2006) Ocular circulation in diabetic patients with coronary artery disease. Graefes Arch Clin Exp Ophthalmol 244:163–169
Patton N, Aslam TM, Macgillivray T, Deary IJ, Dhillon B, Eikelboom RH et al (2006) Retinal image analysis: concepts, applications and potential. Prog Retin Eye Res 25:99–127
Nguyen TT, Kawasaki R, Wang JJ, Kreis AJ, Shaw J, Vilser W et al (2009) Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes Care 32:2075–2080
Gugleta K, Kochkorov A, Waldmann N, Polunina A, Katamay R, Flammer J et al (2012) Dynamics of retinal vessel response to flicker light in glaucoma patients and ocular hypertensives. Graefes Arch Clin Exp Ophthalmol 250:589–594
Garhöfer G, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT (2004) Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol 88:887–891
Lott ME, Slocomb JE, Shivkumar V, Smith B, Gabbay RA, Quillen D et al (2012) Comparison of retinal vasodilator and constrictor responses in type 2 diabetes. Acta Ophthalmol 90:e434–e441
Dorner GT, Garhofer G, Kiss B, Polska E, Polak K, Riva CE et al (2003) Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol 285:H631–H636
Lott ME, Slocomb JE, Shivkumar V, Smith B, Quillen D, Gabbay RA et al (2013) Impaired retinal vasodilator responses in prediabetes and type 2 diabetes. Acta Ophthalmol 91:e462–e469
Kubisz P, Stančiaková L, Staško J, Galajda P, Mokáň M (2015) Endothelial and platelet markers in diabetes mellitus type 2. World J Diab 6:423–431
Lim HS, Chong AY, Freestone B, Blann AD, Lip GY (2005) The effect of multi-factorial intervention on plasma von Willebrand factor, soluble E-selectin and tissue factor in diabetes mellitus: implications for atherosclerotic vascular disease. Diabet Med 22:249–255
Hamilton SJ, Watts GF (2013) Endothelial dysfunction in diabetes: pathogenesis, significance, and treatment. Rev Diabet Stud 10:133–156
Cheng H, Harris RC (2014) Renal endothelial dysfunction in diabetic nephropathy. Cardiovasc Hematol Disord Drug Targets 14:22–33
Gilbert RE (2014) The endothelium in diabetic nephropathy. Curr Atheroscler Rep 16:410. doi:10.1007/s11883-014-0410-8
Landray MJ, Wheeler DC, Lip GY, Newman DJ, Blann AD, McGlynn FJ et al (2014) Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis 43:244–253
Umemura T, Kawamura T, Hotta N, Koga H, Sugiyama S, Kugiyama K, Watanabe K, Fukushima H, Tanaka T, Sakamoto T, Yoshimura M, Jinnouchi H, Ogawa H (2005) Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol 45:1622–1630
Tramontano AF, Lyubarova R, Tsiakos J, Palaia T, Deleon JR, Ragolia L (2010) Circulating endothelial microparticles in diabetes mellitus. Mediat Inflamm 250476. doi:10.1155/2010/250476
Lenting PJ, Christophe OD, Denis CV (2015) von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood 125:2019–2028
Blann AD (2006) Plasma von Willebrand factor, thrombosis, and the endothelium: the first 30 years. Thromb Haemost 95:49–55
Porta M, Townsend C, Clover GM, Nanson M, Alderson AR, McCraw A, Kohner EM (1981) Evidence for functional endothelial cell damage in early diabetic retinopathy. Diabetologia 20:597–601
Lufkin EG, Fass DN, O’Fallon WM, Bowie EJ (1979) Increased von Willebrand factor in diabetes mellitus. Metabolism 28:63–66
Zarbock A, Ley K, McEver RP, Hidalgo A (2011) Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118:6743–6751
Roldán V, Marín F, Lip GY, Blann AD (2003) Soluble E-selectin in cardiovascular disease and its risk factors. Thromb Haemost 90:1007–1020
Gearing AJ, Hemingway I, Pigott R, Hughes J, Rees AJ, Cashman SJ (1992) Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: pathological significance. Ann N Y Acad Sci 667:324–331
Paneni F, Beckman JA, Creager MA, Cosentino F (2013) Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J 34:2436–2443
Kampoli AM, Tousoulis D, Briasoulis A, Latsios G, Papageorgiou N, Stefanadis C (2011) Potential pathogenic inflammatory mechanisms of endothelial dysfunction induced by type 2 diabetes mellitus. Curr Pharm Des 17:4147–4158
Galada P, Martibka E, Mokan M, Kubisz P (1997) Endothelial markers in diabetes mellitus. Thrombosis Res 85:63–65
Chen Y, Feng B, Li X, Ni Y, Lou Y (2012) Plasma endothelial microparticles and their correlation with the presence of hypertension and arterial stiffness in patients with type 2 diabetes. J Clin Hypertens 14:455–460
Yip, WF, Sabanayagam C, Teo BW, Tay WT, Ikram MK, Tai ES et al (2015) Retinal microvascular abnormalities and risk of renal failure in Asian populations. PLOS ONE 10(2):e0118076
Eriksen BO, Lochen ML, Arntzen KA, Bertelsen G, Winther EBA, von Hanno T et al (2015) Estimated and measured GFR associate differently with retinal vasculopathy in the general population. Nephron 131:175–184
Lim LS, Cheung CY, Sabanayagam C, Lim SC, Tai ES, Hung L et al (2013) Structural changes in the retinal microvasculature and renal function. Invest Opthalm Vis Sci 54(4):2970–2976
Acknowledgments
We would like to thank our colleagues Drs. GYH Lip, R. Ryder, D. Nichol, B. Lee, and J. Khan for help in recruitment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The Hans and Gertrude Fund from Fight for Sight provided financial support in the form of research funding. The sponsor had no role in the design or conduct of this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Heitmar, R., Varma, C., De, P. et al. The relationship of systemic markers of renal function and vascular function with retinal blood vessel responses. Graefes Arch Clin Exp Ophthalmol 254, 2257–2265 (2016). https://doi.org/10.1007/s00417-016-3432-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3432-9