Abstract
Purpose
To determine the ocular anatomical factors influencing the pupillary light reactions to different wavelengths of light, measured with chromatic pupillometry.
Methods
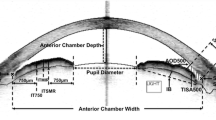
Community-based, cross-sectional study including subjects with normal ocular health (ages 50–79 years). Direct pupillary responses to continuously increasing irradiances (6.8 to 13.8 log photons cm−2 s−1) of red (631 nm) and blue (469 nm) light were measured, using a dedicated infrared pupillometer. All subjects underwent swept source optical coherence tomography (SS-OCT, CASIA SS-1000, Tomey Corporation, Nagoya, Japan) and noncontact partial coherence laser interferometry (Lenstar LS900, Haag-Streit AG, Switzerland). Univariate and multivariable regression analyses were performed to determine the anatomical features influencing pupillographic parameters.
Results
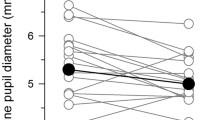
Among the 177 included subjects, 167 (94.4 %) were Chinese and 116 (65.5 %) female. The average baseline pupil diameter in darkness (β = −0.080, p < 0.001) and the amplitude of the relative pupillary constriction (β = −0.233, p = 0.006) to blue light decreased with age. The amplitude of pupillary constriction was significantly larger in patients with a thinner iris, in response to stimulation with blue (β = −0.321, p < 0.001) and red light (β = −0.336, p < 0.001). Other ocular parameters (i.e., lens vault, anterior chamber depth width, iris volume, iris curvature, and lens thickness) were not significantly associated with pupillometric outcomes.
Conclusions
The amplitude of the pupillary light constriction to chromatic photic stimuli is reduced with increasing age and iris thickness in subjects with normal ocular health, a finding which needs to be integrated into future pupillometric studies.
Similar content being viewed by others
References
Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM (2006) Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 20;497(3):326–49
Kankipati L, Girkin CA, Gamlin P (2010) Post-illumination pupil response in subjects without ocular disease. Invest Ophthalmol Vis Sci 51:2764–2769
Girkin CA (2003) Evaluation of the pupillary light response as an objective measure of visual function. Ophthalmol Clin N Am 16(2):143–153
Wyatt HJ, Musselman JF (1981) Pupillary light reflex in humans: evidence for an unbalanced pathway from nasal retina, and for signal cancellation in brain-stem. Vis Res 21(4):513–525
Lowenstein O, Lowenfeld IE (1969) The pupil. In: Davson H (ed) The eye. Academic, New York, pp 255–337
Fry GA (1945) The relation of pupil size to accommodation and convergence. Am J Optom 22:451–465
Frederick W, Kerr L, Hollowell OW (1964) Location of pupillomotor and accommodation fibres in the oculomotor nerve: experimental observations on paralytic mydriasis. J Neurol Neurosurg Psychiatr 27(5):473–481
Hess EH (1972) Pupillometrics: a method of studying mental, emotional, and sensory processes. In: Greenfield NS, Sturnbach RA (eds) Handbook of psychophysiology. Holt, Reinhardt and Winston, New York, pp 491–534
Binda P, Murray SO (2015) Keeping a large-pupilled eye on high-level visual processing. Trends Cogn Sci 19(1):1–3
Smith SA (1992) Pupil function: tests and disorders. In: Bannister R, Mathias CJ (eds) Autonomic failure, 3rd edn. Oxford University Press, Oxford, pp 393–412
Loewenfeld IE (1993) The pupil: anatomy, physiology, and clinical applications. Iowa State University Press/Amos Wayne State University Press, Detroit, pp 407–479, 498–517, 1131–1187, 1200–1203
Herbst K, Sander B, Lund-Andersen H, Broendsted AE, Kessel L, Hansen MS, Kawasaki A (2012). Intrinsically photosensitive retinal ganglion cell function in relation to age: a pupillometric study in humans with special reference to the age-related optic properties of the lens. BMC Ophthalmol 12:4
Chylack LT Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY (1993) The lens opacities classification system III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 111(6):831–836
Leung CK, Weinreb RN (2011) Anterior chamber angle imaging with optical coherence tomography. Eye (Lond) 25(3):261–267
Nongpiur ME, Sakata LM, Friedman DS et al (2010) Novel association of smaller anterior chamber width with angle closure in Singaporeans. Ophthalmology 117:1967–1973
Nongpiur ME, He MG, Amerasinghe N et al (2011) Lens vault, thickness and position in Chinese subjects with angle closure. Ophthalmology 118:474–479
Wang BS, Sakata LM, Friedman DS et al (2010) Quantitative iris parameters and association with narrow angles. Ophthalmology 117(1):11–17
Gooley JJ, Ho Mien I, St Hilaire MA et al (2012) Melanopsin and rod-cone photoreceptors play different roles in mediating pupillary light responses during exposure to continuous light in humans. J Neurosci 32:14242–14253
Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW (2010) Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med 2(31):31ra33
Ortube MC, Kiderman A, Eydelman Y, Yu F, Aguilar N, Nusinowitz S, Gorin MB (2013) Comparative regional pupillography as a noninvasive biosensor screening method for diabetic retinopathy. Invest Ophthalmol Vis Sci 54(1):9–18
Herbst K, Sander B, Lund-Andersen H, Wegener M, Hannibal J, Milea D (2013) Unilateral anterior ischemic optic neuropathy: chromatic pupillometry in affected, fellow non-affected and healthy control eyes. Front Neurol 4:52
Nissen C, Rönnbäck C, Sander B, Herbst K, Milea D, Larsen M, Lund-Andersen H (2015) Dissociation of pupillary post-illumination responses from visual function in confirmed OPA1 c.983A > G and c.2708_2711delTTAG autosomal dominant optic atrophy. Front Neurol 6:5
Tatham AJ, Meira-Freitas D, Weinreb R, Zangwill LM, Medeiros FA (2014) Detecting glaucoma using automated pupillography. Ophthalmology 121(6):1185–1193
Kankipati L, Girkin CA, Gamlin PD (2011) The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci 52:2287–2292
Chang DS, Xu L, Boland MV, Friedman DS (2013) Accuracy of pupil assessment for the detection of glaucoma. Ophthalmology 120(11):2217–2225
Aptel F, Denis P (2010) Optical coherence tomography quantitative analysis of iris volume changes after pharmacologicmydriasis. Ophthalmology 117(1):3–10
Zheng C, Cheung CY, Aung T, Narayanaswamy AK, Ong SH, Friedman DS, Allen JC, Baskaran M, Chew PT, Perera S (2012) In vivo analysis of vectors involved in pupil constriction in Chinese subjects with angle closure. Invest Ophthalmol Vis Sci 53(11):6756–6762
Zheng C, Guzman CP, Cheung CY, He Y, Friedman DS, Ong SH, Narayanaswamy AK, Chew PT, Perera SA, Aung T (2013) Analysis of anterior segment dynamics using anterior segment optical coherence tomography before and after laser peripheral iridotomy. JAMA Ophthalmol 131(1):44–49
Fotiou DF, Brozou CG, Tsiptsios DJ et al (2007) Effect of age on pupillary light reflex: evaluation of pupil mobility for clinical practice and research. Electroyogr Clin Neurophysiol 47(1):11–22
Pfeifer MA, Weinberg CR, Cook D, Best JD, Reenan A, Halter JB (1983) Differential changes of autonomic nervous system function with age in man. Am J Med 75(2):249–258
Lowenfeld IE (1979) Pupillary changes related to age. In: Topics in Neuro-Ophthalmology. Williams & Wilkins, Baltimore, pp 124–150
Bistosis P, Prettyman R, Szabadi E (1996) Changes in autonomic function with age: a study of pupillary kinetics in healthy young and old people. Age Ageing 25:432–438
Pokorny J, Smith VC, Lutze M (1987) Aging of the human lens. Appl Opt 26(8):1437–1440
McDougal DH, Gamlin PD (2010) The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vis Res 50:72–87
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial support
This work was supported by the Duke-NUS Signature Research Program funded by the Agency for Science, Technology and Research, Singapore, and the Ministry of Health, Singapore; National Medical Research Council, Singapore under NMRC/CIRG/1401/2014(DM); National Medical Research Council, Singapore under NMRC/NIG/1000/2009 (JJG); Singapore National Eye Centre Health Research Endowment Fund (1005/20/2013, AT, JJG, DM); Biomedical Research Council under 01-TCRP-2010 (TCR0101674, AT). The sponsor had no role in the design or conduct of this research.
Conflict of interest
Dan Milea and Joshua J Gooley have a patent Application PCT/SG2015/050494: A method and system for monitoring and/or assessing pupillary responses
The rest of authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Sharma, S., Baskaran, M., Rukmini, A.V. et al. Factors influencing the pupillary light reflex in healthy individuals. Graefes Arch Clin Exp Ophthalmol 254, 1353–1359 (2016). https://doi.org/10.1007/s00417-016-3311-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3311-4