Abstract
This study aimed to examine the existing literature that investigated the effectiveness of optical coherence tomography (OCT) and optical coherence tomography angiography (OCT-A) as a biomarker for idiopathic intracranial hypertension (IIH). Our search was conducted on January 17th, 2024, and included the databases, Medline, Scopus, Embase, Cochrane, Latin American and Caribbean Health Sciences Literature (LILACS), International Standard Randomized Controlled Trial Number (ISRCTN) registry, and the International Clinical Trials Registry Platform (ICTRP). Our final review included 84 articles. In 74 studies, OCT was utilized as the primary ocular imaging method, while OCT-A was employed in two studies including eight studies that utilized both modalities. Overall, the results indicated that IIH patients exhibited significant increases in retinal nerve fiber layer (RNFL) thickness, total retinal and macular thickness, optic nerve head volume, and height, optic disc diameter and area, rim area, and thickness compared to controls. A significant correlation was observed between cerebrospinal fluid (CSF) pressure and OCT parameters including RNFL thickness, total retinal thickness, macular thickness, optic nerve head volume, and optic nerve head height. Interventions aimed at lowering CSF pressure were associated with a substantial improvement in these parameters. Nevertheless, studies comparing peripapillary vessel density using OCT-A between IIH patients and controls yielded conflicting results. Our systematic review supports OCT as a powerful tool to accurately monitor retinal axonal and optic nerve head changes in patients with IIH. Future research is required to determine the utility of OCT-A in IIH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic intracranial hypertension (IIH) is a disorder of unknown etiology characterized by raised intracranial pressure (ICP) that predominantly affects obese women of childbearing age [1]. The prevalence of IIH in the general population is 1–3 per 100,000 people but among women of childbearing age, the prevalence rate is higher at 5.5 per 100,000 [2,3,4,5]. The incidence of IIH has increased due to the rapid increase of obesity and the estimated total cost of IIH in the USA alone has exceeded USD $444 million [6]. Although the exact cause of IIH is unknown, several theories have been postulated, including increased abdominal pressure, sleep apnea syndrome, reduction in cerebrospinal fluid (CSF) outflow or elevated venous sinus pressure [7,8,9]. The predominant symptom of IIH is headache, which can vary in intensity from mild to severe [10, 11] and chronic headache has been shown to significantly impact the quality of life of individuals with IIH [12, 13]. Other symptoms of IIH include tinnitus, visual obscuration, and diplopia [1, 10, 14, 15]. The accepted criteria for diagnosis of IIH includes the combination of raised ICP without hydrocephalus or mass lesions, normal CSF composition, and normal neuroimaging [13]. There are currently no evidence-based guidelines for the medical and surgical management of IIH due to a lack of information on the efficacy of treatments and possible side effects [16, 17].
Chronic elevated ICP can lead to cerebral ischemia, cerebral edema, herniation, irreversible brain damage and in severe cases, death [18]. Hence, the precise measurement and continuous monitoring of ICP are crucial in caring for patients with IIH [19]. ICP can be accurately assessed using lumbar and transcranial methods but are invasive and carry an increased risk of bleeding and infection [20, 21]. Several non-invasive methods such as magnetic resonance imaging, computerized axial tomography imaging, transcranial doppler ultrasonography, tympanic membrane displacement, and ocular ultrasound have also been used to monitor the ICP changes in patients with raised IIH. However, these methods have limitations such as low sensitivity and specificity, poor inter-rater reliability, and poor test predictability [22]. Therefore, more accurate and reliable biomarkers are needed to evaluate the disease state.
Optical coherence tomography (OCT) and optical coherence tomography angiography (OCT-A) are imaging modalities that provides qualitative and quantitative evaluation of the changes in the retinal nerve fibre layer (RNFL), optic nerve head, macula, and retinal and choroidal perfusion. While OCT can provide structural information about the retina and choroid, OCT-A provides information about the vasculature and blood flow of the retina and choroid (Table 1). OCT/OCT-A are routinely used in ophthalmic clinical settings to diagnose and monitor retinal conditions such as diabetic retinopathy, age-related macular degeneration, and retinal vascular occlusions [23,24,25,26,27,28,29]. OCT allows visualization of optic nerve head swelling, changes in the RNFL and retinal pigment epithelium/Bruch’s membrane (RPE/BM) layer associated with acute and chronic changes in ICP. In addition, OCT-A allows the evaluation of vessel density on the optic disc and peripapillary region in both newly diagnosed and chronic IIH cases, making these imaging modalities valuable tools for both diagnosing and monitoring IIH [30, 31]. OCT can be useful to differentiate true disc edema, including papilledema from pseudoedema due to optic disc drusen. Studies have shown that RNFL thickness particularly in the nasal and inferior quadrants were reduced in optic disc drusen compared to optic disc edema [32, 33]. The standard for evaluating the severity of papilledema is the Frisén scale which grades the optic disc swelling from 0 to 5. Three-dimensional OCT parameters such as optic nerve head volume, height, and shape could potentially offer greater sensitivity compared to the Frisén scale in evaluating treatment outcomes among IIH patients [34]. This is because even in IIH patients with normal RNFL thickness, the optic nerve head volume has been shown to be elevated [35]. The configuration of the RPE/BM layer in OCT scans can aid in distinguishing papilledema from disc edema caused by other factors like anterior ischemic optic neuropathy (AION). In papilledema, the RPE/BM layer exhibits a U-shape, angled toward the vitreous, whereas in AION and normal individuals, it assumes a V-shaped configuration, angled away from the vitreous [36, 37]. Increased ICP in IIH can cause biomechanical stress on the optic nerve head and retina resulting in retinal and choroidal folds, and OCT has been shown to be more sensitive than fundus photography is detecting these folds [38]. Quantitative assessments of vessel density surrounding the optic nerve head have shown a reduction in disorders such as optic neuritis, arteritic anterior ischemic optic neuropathy (AAION), and optic atrophy [39]. As such, the usefulness of OCT-A in diagnosing and monitoring IIH is still unclear. This systematic review examined the current body of literature regarding the utilization of OCT/OCT-A as a biomarker for IIH and reports the most suitable OCT/OCT-A parameters for the diagnosis and monitoring of IIH.
Methods
This systematic review followed the reporting guidelines outlined in the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) and was registered with the International Prospective Register of Systematic Reviews (PROSPER; ID: CRD42024520282). An initial search was performed using Medline and CINAHL to identify relevant articles and keywords. An extensive search strategy was then developed in Medline based on the identified keywords and index terms. The keywords used for the search included: “idiopathic intracranial hypertension”, “pseudotumor cerebri”, “pseudotumor syndrome”, “Nonne’s syndrome”, “otitis hydrocephalus”, “benign intracranial hypertension”, “non-infective serous meningitis”. The retrieved articles from the Medline search were evaluated to confirm the inclusion of key publications. The search strategy, including the keywords and index terms, were adapted for other bibliographic databases such as PsycINFO (via Ovid SP), Latin American and Caribbean Health Sciences Literature (LILACS) and Scopus (Elsevier) (Online Resource 1). Each database search strategy was run on 17 January 2024. In addition, grey literature sources were searched including the International Standard Randomized Controlled Trial Number (ISRCTN) registry and the International Clinical Trials Registry Platform (ICTRP). There were no limits applied to language, but studies were excluded if they were solely animal studies, case report/case series, editorials, reviews, or conference abstracts. The primary outcome was to report the retinal and optic nerve head changes using OCT/OCT-A in IIH patients. Two authors (MPS and JE) independently evaluated the titles and abstracts and then full-text reports for relevance utilizing Covidence (IBM, Detroit USA). Any discrepancies in the screening were resolved by mutual consensus between the authors. Reasons for exclusion of the studies were reported in each step of the review process. Included studies were assessed for quality according to the National Institutes of Health Quality Assessment Tool for observational cohort, case–control, cross-sectional, before–after studies with no control groups and controlled intervention studies. The assessment of case–control and before–after studies included 12 items scored as “yes”, “no”, or “other” (cannot determine, not applicable, not reported). The assessment of cohort, observational, and controlled intervention studies included 14 items that were again scored as “yes”, “no” or “other” (cannot determine, not applicable, not reported).
Results
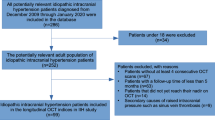
Our initial search yielded 852 articles. Upon reviewing titles and abstracts, 718 articles were excluded (Fig. 1). After examining the full text of the remaining articles, an additional 50 were excluded. The final review comprised 84 articles that utilized ocular imaging as a biomarker for IIH (Table 2). We categorized the included articles into two groups: (1) studies employing OCT as the imaging technique in IIH, and (2) studies employing OCT-A as the imaging technique in IIH. The assessment of the risk of bias of the studies included in this review is shown in Online Resource 2.
Studies investigating OCT as the imaging modality in IIH
There were 82 studies that used OCT as an imaging modality in IIH. In these studies, OCT imaging was used to evaluate treatment effectiveness in patients with IIH, to compare the retinal and optic nerve head changes between IIH patients and healthy controls or to ascertain the relationship between OCT measurements and clinical parameters. The following OCT parameters were compared among the studies that reported them: peripapillary RNFL thickness [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63], total retinal thickness [34, 64,65,66,67,68,69,70,71], macular thickness [72,73,74,75], macular ganglion cell complex (GCC) thickness [76,77,78,79,80,81,82,83], ganglion cell layer thickness [70, 84, 85], optic nerve head shape [30], optic nerve head volume [34, 35, 86,87,88], optic nerve head height [7, 35], optic nerve protrusion length [89], optic disc area [90, 91], optic disc diameter [92, 93], rim area [90, 91], rim thickness [50], optic cup volume [90], retinal folds [37, 94, 95], shape of peripapillary retinal pigment epithelium-basement membrane (ppRPE/BM) layer [7, 19, 30, 36, 96,97,98], anterior laminar cribrosa surface depth [99], posterior lamina cribrosa surface depth [99, 100], lamina cribrosa thickness [99, 100], Bruch’s membrane opening [69, 100], and pre-laminar tissue thickness (Figs. 2 and 3) [100]. The RNFL thickness was the OCT parameter most used in these studies [40, 43, 64, 66]. Most studies that compared the RNFL thickness between IIH patients and age-matched controls demonstrated that IIH patients had a significantly greater RNFL thickness compared to the controls [40, 43, 44, 51, 63, 64, 66, 101]. However, one study contradicted this trend, suggesting that control subjects had higher RNFL thickness compared to those with IIH, while another study found no significant difference in RNFL thickness between IIH patients and controls [81, 90]. In addition, it was observed that that IIH patients had initially thicker RNFL measurements, which gradually decreased over subsequent follow-up periods at 1, 3, 6, and 12 months [34, 40, 66, 67]. Among the IIH patients, those with severe papilledema were shown to have thicker RNFL than in patients with normal optic discs/minimally or moderately raised discs [41]. Patients with recurrent IIH and those without recurrence of IIH were found to have significantly different RNFL thicknesses, with the recurrence group reported to have thicker neural tissue [91]. Similarly, RNFL was greater in papilledema than in pseudopapilledema patients [54]. However, the RNFL thickness did not differ between the symptomatic and asymptomatic groups [85]. Compared to healthy controls, patients with chronic and atrophic papilledema had significantly thinner RNFL thickness [102]. In OCT imaging, ppRPE/BM is seen as a well-defined layer above the choroid in the outer retina. It is V-shaped and angled away from the vitreous in normal individuals (Fig. 4). However, in IIH patients with raised ICP, it is U-shaped and angled toward the vitreous (Fig. 5) [36, 98]. Interventions aimed at lowering the CSF pressure such as lumbar puncture and CSF shunt have demonstrated an ability to transform the ppRPE/BM layer from a U-shaped configuration to the more typical V-shaped configuration [19, 30, 36].
Optical coherence tomography (OCT) image of the retina showing the different layers. ILM inner limiting membrane; RNFL retinal nerve fiber layer; GCL ganglion cell layer; INL inner nuclear layer; IPL inner plexiform layer; ONL outer nuclear layer; OPL outer plexiform layer; ELM external limiting membrane; RPE retinal pigment epithelium. Macular thickness = distance between ILM and RPE; retinal thickness = distance between ILM and photoreceptor layer; choroidal thickness = distance between the outer border of RPE and choroidoscleral surface
A Optical coherence tomography (OCT) image of the optic nerve head. In healthy individuals, the peripapillary retinal pigment epithelium-basement membrane (ppRPE/BM) is V-shaped and angled away from the vitreous. B Optical coherence tomography (OCT) image of the optic nerve head showing a U-shape configuration of the peripapillary retinal pigment epithelium-basement layer (ppRPE/BM) in a patient with papilledema. C Optical coherence tomography angiography (OCT-A) image of the optic nerve head
Optical coherence tomography angiography (OCT-A) image displays the segmentation of three capillary plexus, including superficial, deep, and choriocapillary plexus, both in en face (top row) and cross-sectional (bottom row). The segmentation boundaries for each layer are indicated by a pink line on the cross-sectional OCT-A image
Few studies used a custom segmentation algorithm to develop 3D parameters such as optic nerve head volume and optic nerve head height to compare the optic nerve head changes between IIH patients and controls. They showed that the optic nerve head volume and optic nerve head height were increased in IIH patients than in controls [35, 103]. In addition, optic nerve head volume was used to differentiate between controls, treated, and untreated patients with IIH [35]. Optic disc area, diameter, rim area, thickness, and Bruch’s membrane opening were reported to be thicker in IIH patients compared to controls [69, 90]. In contrast, individuals with IIH exhibited thinner macular GCC, diminished thickness of the peripapillary choroid, reduced depth of both the anterior and posterior lamina cribrosa surfaces depths, and a decrease in optic cup volume [42, 77, 81, 90, 99]. OCT imaging was also shown to be sensitive in detecting folds such as peripapillary wrinkles, retinal and choroidal folds in patients with IIH [95].
Studies that investigated the utility of OCT in evaluating treatment outcomes in IIH patients demonstrated a significant improvement in the OCT parameters (RNFL thickness, total retinal thickness, choroidal thickness, optic nerve head volume, rim, and disc area) after interventions such as weight loss, oral acetazolamide, and optic nerve sheath fenestration [45, 86, 91]. Studies that examined the relationship between OCT measurements and clinical parameters showed a significant positive correlation between CSF pressure and various OCT parameters, including RNFL thickness, retinal thickness, macular GCC, optic nerve head volume, optic nerve head height, ppRPE/BM layer and Bruch’s membrane opening [35, 50, 51, 59, 61, 69, 86]. RNFL thickness showed significant positive correlation with visual acuity, visual field loss, papilledema severity, and the Modified Frisén Scale (MFS grades) from fundus photographs [40, 65, 77]. In addition, macular GCC thickness was found to be significantly associated with optic disc pallor [81]. However, no association was identified between CSF pressure and the shape of the optic nerve head [97].
Studies investigating OCT-A as the imaging modality in IIH
Ten studies explored the applicability of OCT-A as a non-invasive imaging biomarker for IIH. In these studies, OCT-A examinations were primarily done in acute settings of papilledema except two studies in chronic papilledema settings. Most of these studies employed OCT-A to assess the peripapillary vascular density differences between individuals with IIH and control groups. The peripapillary area is a ring-shaped zone extending from the optic disc boundary (Fig. 4) [104]. Vessel density is defined as the percentage of area occupied by both large vessels and microvasculature in a specific area and is calculated over the entire scan area, as well as in the defined sectors within the scan [105]. Capillary flux intensity is defined as the total weighted area of perfused microvascular per unit area and capillary perfusion density is defined as the total area of perfused microvasculature per unit area. The retinal vascular network is organized into four distinct plexuses: the superficial capillary plexus (SCP), intermediate capillary plexus (IP), deep capillary plexus (DCP), and radial peripapillary capillary plexus (RPC) [106]. The central retinal artery supplies blood to the SCP which then anastomoses and creates the IP and the DCP. The SCPs are located with the RNFL, ganglion cell layer, and the inner plexiform layer and the DCPs are located within the outer plexiform layer below the IP. The RCP, however, runs parallel with the nerve fiber layer axons (Fig. 5).
Several studies have investigated the peripapillary vessel density in patients with IIH in comparison to controls. Tuntas et al. found a significant reduction in peripapillary vessel density among IIH patients compared to controls in their study using the AngioVue OCT-A device, which exclusively reported peripapillary vessel density (both global and sectoral). [107] Similarly, Cakir et al., utilizing Topcon imaging, observed a notable decrease in peripapillary vessel density across different retinal capillary plexus (SCP, DCP, and choriocapillaries) in IIH patients compared to controls [108]. However, Kaya et al., also using AngioVue reported a significant elevation in peripapillary vessel density in IIH patients compared to controls, offering a contradictory perspective [109]. In contrast, Fard et al., employing AngioVue found no significant difference in peripapillary capillary density between papilledema patients and controls [110]. Moreover, microvascular densities showed an increase in the nerve fiber layer plexus (NFLP) but a reduction in the SCP and DCP in IIH patients compared to controls using the Svision imaging OCT-A device [111]. Chonsui et al.in their study using PLEX Elite device showed a decreased peripapillary capillary density without changes in capillary flux intensity in eyes with papilledema [112].
Wang et al.in their study using AngioVue device showed that NFLP positively correlated with Frisén scores of patients with IIH [111]. However, SVP, IP, and DCP inversely correlated with Frisén scores of patients with IIH. Similarly, Pahuja et al. showed a negative correlation between superficial peripapillary retinal vessel perfusion and grades of papilledema using the Angioplex device (reported superficial capillary retinal vessel perfusion, deep retinal vessel perfusion and peripapillary choriocapillary perfusion) [102]. Kwapong et al. showed microvascular densities (superficial vascular complex and deep vascular complex) positively correlated with ICP using the Svision OCT-A device [113]. Peripapillary capillary vessel density in DCP was significantly reduced in optic disc edema compared to the control group, a condition that can mimic IIH. [108]
Discussion
OCT and OCT-A are non-invasive imaging methods widely used in ophthalmology to provide high-resolution cross-sectional images of the retina [114, 115]. OCT and OCT-A measurements have also shown to be a reliable indicator of neuronal death in various neurological disorders such as Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, neuromyelitis optica, and spinocerebellar ataxia [116,117,118,119]. This systematic review examined existing literature to assess the effectiveness of OCT/OCT-A as a diagnostic and monitoring modality for IIH. The predominant imaging technique in the reviewed studies was OCT, with only ten studies using OCT-A. Among studies using OCT as the imaging modality for IIH patients, the most frequently assessed parameter was RNFL thickness. Conversely, studies employing OCT-A as the imaging modality for IIH patients predominantly focused on peripapillary vessel density. In summary, studies utilizing OCT revealed increased thickness in RNFL, retina, as well as increased measurements in optic nerve head volume, optic nerve head height, optic disc diameter, rim area, and rim thickness. However, studies that used OCT-A as the imaging modality showed conflicting results regarding the peripapillary vessel density.
The RNFL comprises axons originating from retinal ganglion cells that converge from the retina and macular region to form the optic nerve. The RNFL is visualized in OCT images as the inner most retinal layer beneath the internal limiting membrane (Fig. 2). The peripapillary RNFL is measured along a 3 mm diameter circle centered on the optic nerve head and the mean thickness of the upper retinal layer is then presented as the average RNFL thickness (Fig. 6). The increased RNFL thickness reported in individuals with IIH is due to the disruption of the axonal transport and intraneural optic nerve sheath ischemia caused by the elevated CSF pressure in the subarachnoid space surrounding the optic nerve. Conversely, the reported decrease in macular GCC thickness in IIH patients is due to the loss of nerve fibers and retinal ganglion cells resulting from oxidative stress-associated prolonged swelling. The subarachnoid space connected to the optic nerve sheath undergoes structural changes due to alterations in the translaminar pressure gradient (difference between intraocular pressure and CSF pressure). Elevated ICP compresses the retrolaminar optic nerve and peripapillary scleral flange, causing deformation of the ppRPE/BM and adjacent sclera toward the vitreous [120,121,122]. Studies in this review have shown that in IIH patients, the configuration of the ppRPE/BM follows a U-shaped pattern around the optic nerve head, transitioning to a V-shape after interventions to lower CSF levels [19].
The earliest finding of raised ICP is optic disc swelling which takes about a week or 10 days to appear. However various diagnostic methods such as MRI, serum hormonal assay, axial length evaluation, pattern electroretinogram (PERG), and visually evoked potential (VEP) tests, can aid in detecting subclinical IIH. Liu et al. demonstrated that patients experiencing pulsatile tinnitus displayed several ocular and intracranial signs of IIH on MRI scans, such as optic nerve sheath enlargement, optic nerve tortuosity, posterior globe flattening, empty Sella, downward displacement of cerebellar tonsils into the foramen magnum, and slit-like lateral ventricles [123].According to a study by Prabhat and colleagues, hormonal abnormalities such as raised prolactin, decreased TSH, and decreased cortisol were found in 37.5% of patients with IIH [124]. Moreover, studies have shown that the mean PERG and VEP amplitudes were reduced in IIH patients compared to healthy individuals [125]. Madill et al.in their study reported a significant difference in globe shape and axial length between patients with IIH and control subjects [126].
Swelling of the optic disc and increased thickness of the RNFL are not exclusive indicators of IIH or increased ICP because they can also occur in other optic neuropathies like optic neuritis and AION. Nevertheless, various parameters that describe the shape of the optic nerve head, such as, optic nerve head volume, optic nerve cup volume, central optic nerve head thickness, volume of Bruch’s membrane opening region, bending energy, minimal rim width of Bruch’s membrane opening (BMO-MRW), surface area of BMO-MRW, and area of Bruch’s membrane opening, may aid in distinguishing between different optic neuropathies. In a study by Yadav et al., a 3D model of the optic nerve head was constructed using high-resolution OCT volume scans, and it was demonstrated that all of the aforementioned parameters, except for bending energy, exhibited differences between IIH, healthy controls, and optic neuritis [127]. Similarly, Kaufhold and colleagues employed volume scans to gauge optic nerve head volume in their study, revealing that 3D parameters such as optic nerve head volume and height could distinguish between IIH patients and controls [35]. These parameters were shown to be elevated in IIH patients even when the RNFL showed normal thickness, suggesting that it could serve as a potential marker of treatment efficacy and disease advancement [35]. Future studies employing OCT as a diagnostic tool for IIH could utilize the 3D optic nerve head parameters to differentiate IIH for other optic neuropathies such as glaucoma, optic neuritis, and AION.
The central retinal artery and ophthalmic artery traverse through the subarachnoid space and are influenced by changes in ICP [128]. Out of ten studies utilizing OCT-A as an imaging modality in IIH, five revealed a decrease in peripapillary vessel density [107, 108, 111,112,113], one demonstrated an increase in vessel density [109], and one found no disparity in vessel density between IIH patients and controls [110]. The decrease in vessel density seen in OCT-A can be due to mechanical compression of the capillary network caused by elevated ICP [129] or due to artifacts arising from the shadowing effect of fluid in papilledema artificially leading to decreased capillary density [129, 130]. Reduction in capillary vessel density has also been reported in other acute and chronic optic neuropathies such as optic neuritis, Leber’s hereditary optic neuropathy (LHON), optic atrophy and non-arteritic anterior ischemic optic neuropathy (NAION) [39]. In cases of optic neuritis and dominant optic atrophy, the decrease in vessel density may result from reduced metabolic demands caused by neuronal degeneration and the atrophy of the peripapillary RNFL and GC-IPL, which subsequently reduces blood flow through autoregulatory mechanisms [131]. However, LHON is a peripapillary microangiopathy that affects the endothelial and smooth muscle components of the blood vessel walls causing a significant reduction in the peripapillary capillary density [132]. In NAION, ischemic alterations due to dysfunctional vascular autoregulation may lead to the destruction of the capillaries [39].
The OCT parameters such as RNFL thickness, macular GCC thickness, rim area, disc area, and cup volume are easily obtained through device software (Figs. 6 and 7). However, certain parameters like optic nerve head volume, optic nerve head height, optic nerve head shape, peripapillary Bruch’s membrane angle, anterior laminar surface depth, posterior laminar surface depth, and Bruch’s membrane opening require manual calculation using custom segmentation algorithms. Most OCT devices do not automatically provide these measures, limiting their practicality in routine clinical use. In addition, accurately segmenting the outer retinal boundary in the presence of papilledema can be challenging and may lead to inaccuracies [133]. Another crucial OCT parameter for distinguishing papilledema in IIH from optic disc edema caused by other factors is the ppRPE/BM shape changes. These changes have shown a correlation with ICP [96]. However, the practical application of using ppRPE/BM changes in guiding clinical therapy is hindered by the lack of a commercial method and the need for extensive image processing to identify the RPE/BM boundary beneath an enlarged optic nerve head, limiting the integration of ppRPE/BM changes into clinical decision-making. OCT-A is relatively newer imaging methods offering both structural and blood flow information within the retina and the choroid [134]. Given that recent studies using OCT-A in IIH have produced inconsistent findings regarding peripapillary vessel density, future research should concentrate on changes in the perifoveal capillary network. This is because IIH is linked to reduced blood flow in the ophthalmic and central retinal arteries [135].
This is the first systematic review to explore studies utilizing ocular imaging as a biomarker for IIH. One of the strengths is that it included 84 studies that used either OCT or OCT-A as the imaging modality in IIH patients. In addition, this systematic review employed study quality assessment tools to appraise the quality of the included studies. There were some limitations in the study. Most of the studies included in this review were case–control studies, with only two randomized control trials. This is because most studies focused on comparing the retinal and optic nerve head changes between IIH patients and healthy controls, thus adopting a case–control design. Another limitation of the study was that it only reviewed various OCT/OCT-A parameters in IIH and could not perform a meta-analysis of these common parameters. This initial step was necessary to understand the current studies and their reporting methods, which will facilitate future meta-analyses. Moreover, conducting a meta-analysis was not possible at this stage given the following reasons: (1) the studies had significantly different designs, methods, and levels of rigor, it might be inappropriate or misleading to statistically combine their results, (2) the outcomes were reported in diverse ways, making it difficult to aggregate the results meaningfully, and (3) some studies lacked sufficient data points for a robust meta-analysis, and some were of low quality or had a high risk of bias, which could lead to misleading conclusions if combined. A narrative approach allows for a more nuanced discussion of the quality and implications of each study. Finally, the specific research question or objective of the review was thought to be better addressed through a narrative synthesis, particularly because the question is broad or exploratory in nature. Another limitation was that our review comprised just ten studies examining the utility of OCT-A in individuals with IIH. This limited number of studies may be attributed to the fact that OCT-A is a relatively recent technology, and its effectiveness in a systemic condition like IIH has yet been firmly established.
To conclude, several OCT parameters have been demonstrated to be different in IIH patients compared to controls and retinal imaging may be useful as an efficient, non-invasive, and affordable biomarker for IIH patients.
Data availability
The data supporting the findings of this systematic review can be obtained from the corresponding author, Mallika Prem Senthil on request.
References
Markey KA, Mollan SP, Jensen RH, Sinclair AJ (2016) Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol 15:78–91
Kesler A, Gadoth N (2001) Epidemiology of idiopathic intracranial hypertension in Israel. J Neuroophthalmol 21:12–14
Raoof N, Sharrack B, Pepper IM, Hickman SJ (2011) The incidence and prevalence of idiopathic intracranial hypertension in Sheffield, UK. Eur J Neurol 18:1266–1268
Durcan FJ, Corbett JJ, Wall M (1988) The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol 45:875–877
Craig JJ, Mulholland DA, Gibson JM (2001) Idiopathic intracranial hypertension; incidence, presenting features and outcome in Northern Ireland (1991–1995). Ulster Med J 70:31–35
Friesner D, Rosenman R, Lobb BM, Tanne E (2011) Idiopathic intracranial hypertension in the USA: the role of obesity in establishing prevalence and healthcare costs. Obes Rev 12:e372-380
Jacobson DM, Karanjia PN, Olson KA, Warner JJ (1990) Computed tomography ventricular size has no predictive value in diagnosing pseudotumor cerebri. Neurology 40:1454–1455
Bercaw BL, Greer M (1970) Transport of intrathecal 131-I risa in benign intracranial hypertension. Neurology 20:787–790
Riggeal BD, Bruce BB, Saindane AM, Ridha MA, Kelly LP, Newman NJ, Biousse V (2013) Clinical course of idiopathic intracranial hypertension with transverse sinus stenosis. Neurology 80:289–295
Giuseffi V, Wall M, Siegel PZ, Rojas PB (1991) Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): a case–control study. Neurology 41:239–244
Wall M, George D (1991) Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain 114(Pt 1A):155–180
Mulla Y, Markey KA, Woolley RL, Patel S, Mollan SP, Sinclair AJ (2015) Headache determines quality of life in idiopathic intracranial hypertension. J Headache Pain 16:521
Digre KB, Bruce BB, McDermott MP, Galetta KM, Balcer LJ, Wall M (2015) Quality of life in idiopathic intracranial hypertension at diagnosis: IIH treatment trial results. Neurology 84:2449–2456
Wall M, Kupersmith MJ, Kieburtz KD, Corbett JJ, Feldon SE, Friedman DI, Katz DM, Keltner JL, Schron EB, McDermott MP (2014) The idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA Neurol 71:693–701
Sadun AA, Currie JN, Lessell S (1984) Transient visual obscurations with elevated optic discs. Ann Neurol 16:489–494
Lueck C, McIlwaine G (2005) Interventions for idiopathic intracranial hypertension. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003434.pub2
Biousse V, Bruce BB, Newman NJ (2012) Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry 83:488–494
Smith M (2008) Monitoring intracranial pressure in traumatic brain injury. Anesth Analg 106:240–248
Sibony P, Kupersmith MJ, Honkanen R, Rohlf FJ, Torab-Parhiz A (2014) Effects of lowering cerebrospinal fluid pressure on the shape of the peripapillary retina in intracranial hypertension. Invest Ophthalmol Vis Sci 55:8223–8231
Saladino A, White JB, Wijdicks EF, Lanzino G (2009) Malplacement of ventricular catheters by neurosurgeons: a single institution experience. Neurocrit Care 10:248–252
Gardner PA, Engh J, Atteberry D, Moossy JJ (2009) Hemorrhage rates after external ventricular drain placement. J Neurosurg 110:1021–1025
Raboel PH, Bartek J Jr, Andresen M, Bellander BM, Romner B (2012) Intracranial pressure monitoring: invasive versus non-invasive methods-a review. Crit Care Res Pract 2012:950393
Tran K, Pakzad-Vaezi K (2018) Multimodal imaging of diabetic retinopathy. Curr Opin Ophthalmol 29:566–575
Le P, Zehden J, Zhang AY (2021) Role of optical coherence tomography angiography imaging in patients with diabetes. Curr Diab Rep 21:42
Schimel AM, Fisher YL, Flynn HW Jr (2011) Optical coherence tomography in the diagnosis and management of diabetic macular edema: time-domain versus spectral-domain. Ophthal Surg Lasers Imaging 42(Suppl):S41-55
Regatieri CV, Branchini L, Duker JS (2011) The role of spectral-domain OCT in the diagnosis and management of neovascular age-related macular degeneration. Ophthal Surg Lasers Imaging 42(Suppl):S56-66
Talks SJ, Aftab AM, Ashfaq I, Soomro T (2017) The role of new imaging methods in managing age-related macular degeneration. Asia Pac J Ophthalmol (Phil) 6:498–507
Hirano Y, Suzuki N, Tomiyasu T, Kurobe R, Yasuda Y, Esaki Y, Yasukawa T, Yoshida M, Ogura Y (2021) Multimodal imaging of microvascular abnormalities in retinal vein occlusion. J Clin Med 10:405
Coffey AM, Hutton EK, Combe L, Bhindi P, Gertig D, Constable PA (2021) Optical coherence tomography angiography in primary eye care. Clin Exp Optom 104:3–13
Wang JK, Kardon RH, Ledolter J, Sibony PA, Kupersmith MJ, Garvin MK (2017) Peripapillary retinal pigment epithelium layer shape changes from acetazolamide treatment in the idiopathic intracranial hypertension treatment trial. Invest Ophthalmol Vis Sci 58(5):2554–2565
Anand A, Pass A, Urfy MZ, Tang R, Cajavilca C, Calvillo E, Suarez JI, Venkatasubba Rao CP, Bershad EM (2016) Optical coherence tomography of the optic nerve head detects acute changes in intracranial pressure. J Clin Neurosci 29:73–76
Sarac O, Tasci YY, Gurdal C, Can I (2012) Differentiation of optic disc edema from optic nerve head drusen with spectral-domain optical coherence tomography. J Neuroophthalmol 32:207–211
Carta A, Mora P, Aldigeri R, Gozzi F, Favilla S, Tedesco S, Calzetti G, Farci R, Barboni P, Bianchi-Marzoli S, Fossarello M, Gandolfi S, Sadun AA (2018) Optical coherence tomography is a useful tool in the differentiation between true edema and pseudoedema of the optic disc. PLoS ONE 13:e0208145
Auinger P et al (2015) Papilledema outcomes from the optical coherence tomography substudy of the idiopathic intracranial hypertension treatment trial. Ophthalmology 122:1939-1945.e1932
Kaufhold F, Kadas EM, Schmidt C, Kunte H, Hoffmann J, Zimmermann H, Oberwahrenbrock T, Harms L, Polthier K, Brandt AU, Paul F (2012) Optic nerve head quantification in idiopathic intracranial hypertension by spectral domain OCT. PLoS ONE 7:e36965
Sibony P, Kupersmith MJ, Rohlf FJ (2011) Shape analysis of the peripapillary RPE layer in papilledema and ischemic optic neuropathy. Invest Ophthalmol Vis Sci 52:7987–7995
Kupersmith MJ, Sibony PA, Feldon SE, Wang JK, Garvin M, Kardon R (2017) The effect of treatment of idiopathic intracranial hypertension on prevalence of retinal and choroidal folds. Am J Ophthalmol 176:77–86
Sibony PA, Kupersmith MJ (2016) “Paton’s folds” revisited: peripapillary wrinkles, folds, and creases in papilledema. Ophthalmology 123:1397–1399
Ghasemi Falavarjani K, Tian JJ, Akil H, Garcia GA, Sadda SR, Sadun AA (2016) Swept-source optical coherence tomography angiography of the optic disk in optic neuropathy. Retina 36(Suppl 1):S168-s177
Rebolleda G, Munoz-Negrete FJ (2009) Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci 50:5197–5200
Waisbourd M, Leibovitch I, Goldenberg D, Kesler A (2011) OCT assessment of morphological changes of the optic nerve head and macula in idiopathic intracranial hypertension. Clin Neurol Neurosurg 113:839–843
Marzoli SB, Ciasca P, Curone M, Cammarata G, Melzi L, Criscuoli A, Bussone G, D’Amico D (2013) Quantitative analysis of optic nerve damage in idiopathic intracranial hypertension (IIH) at diagnosis. Neurol Sci 34(Suppl 1):S143-145
Skau M, Yri H, Sander B, Gerds TA, Milea D, Jensen R (2013) Diagnostic value of optical coherence tomography for intracranial pressure in idiopathic intracranial hypertension. Graefes Arch Clin Exp Ophthalmol 251:567–574
Fard MA, Fakhree S, Abdi P, Hassanpoor N, Subramanian PS (2014) Quantification of peripapillary total retinal volume in pseudopapilledema and mild papilledema using spectral-domain optical coherence tomography. Am J Ophthalmol 158:136–143
Starks V, Gilliland G, Vrcek I, Gilliland C (2016) Effect of optic nerve sheath fenestration for idiopathic intracranial hypertension on retinal nerve fiber layer thickness. Orbit 35:87–90
Dinkin MJ, Patsalides A (2017) Venous sinus stenting in idiopathic intracranial hypertension: results of a prospective trial. J Neuroophthalmol 37:113–121
Saenz R, Cheng H, Prager TC, Frishman LJ, Tang RA (2017) Use of A-scan ultrasound and optical coherence tomography to differentiate papilledema from pseudopapilledema. Optom Vis Sci 94:1081–1089
Aojula A, Mollan SP, Horsburgh J, Yiangou A, Markey KA, Mitchell JL, Scotton WJ, Keane PA, Sinclair AJ (2018) Segmentation error in spectral domain optical coherence tomography measures of the retinal nerve fibre layer thickness in idiopathic intracranial hypertension. BMC Ophthalmol 17:257
Huang-Link Y, Eleftheriou A, Yang G, Johansson JM, Apostolou A, Link H, Jin YP (2019) Optical coherence tomography represents a sensitive and reliable tool for routine monitoring of idiopathic intracranial hypertension with and without papilledema. Eur J Neurol 26:808-e857
Onder H, Erkan E (2019) The association of optical coherence tomography results with neuroimaging signs and some clinical parameters in idiopathic intracranial hypertension. J Neurol Res 9:65–71
Merticariu CI, Balta F, Merticariu A, Ciuluvica R, Voinea L (2019) Optical coherence tomography assessment of structural changes in the optic nerve head and peripapillary retina in idiopathic intracranial hypertension. Arch Balk Med Union 54:267–273
Wall M, Subramani A, Chong LX, Galindo R, Turpin A, Kardon RH, Thurtell MJ, Bailey JA, Marin-Franch I (2019) Threshold static automated perimetry of the full visual field in idiopathic intracranial hypertension. Invest Ophthalmol Vis Sci 60:1898–1905
Bahnasy WS, El-Heneedy YAE, Elhassanien MEM, Sharaf AF, Khalid HA (2019) Neuro-ophthalmological biomarkers of visual outcome in newly diagnosed idiopathic intracranial hypertension. Egypt J Neurol Psychiatry Neurosurg 55:26
Flowers AM, Longmuir RA, Liu Y, Chen Q, Donahue SP (2021) Variability within optic nerve optical coherence tomography measurements distinguishes papilledema from pseudopapilledema. J Neuroophthalmol 41(4):496–503
Carey AR, Bosley TM, Miller NR, McCulley TJ, Henderson AD (2021) Use of en face optical coherence tomography to monitor papilledema in idiopathic intracranial hypertension: a pilot study. J Neuroophthalmol 41:212–216
Kohli AA, Pistilli M, Alfaro C, Ross AG, Jivraj I, Bagchi S, Chan J, May D, Liu GT, Shindler KS, Tamhankar MA (2021) Role of ocular ultrasonography to distinguish papilledema from pseudopapilledema. J Neuroophthalmol 41:206–211
Inam ME, Martinez-Gutierrez JC, Kole MJ, Sanchez F, Lekka E, Truong VTT, Lopez-Rivera V, Sheriff FG, Zima LA, Pedroza C, Tang R, Adesina OO, Engstrom A, Sheth SA, Chen PR (2022) Venous sinus stenting for low pressure gradient stenoses in idiopathic intracranial hypertension. Neurosurgery 91:734–740
Thaller M, Tsermoulas G, Sun R, Mollan SP, Sinclair AJ (2021) Negative impact of COVID-19 lockdown on papilloedema and idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry 92:795–797
Sood G, Samanta R, Kumawat D, Agrawal A, Singh A (2022) Clinical profile and retinal nerve fibre layer thickness of optic disc oedema patients at a tertiary care institute in North India. Therapy 14:25158414211072630
Vosoughi AR, Margolin EA, Micieli JA (2022) Idiopathic intracranial hypertension: incidental discovery versus symptomatic presentation. J Neuroophthalmol 42:187–191
Kaya Tutar N, Kale N (2023) The relationship between lumbar puncture opening pressure and retinal nerve fiber layer thickness in the diagnosis of idiopathic intracranial hypertension: is a lumbar puncture always necessary? Neurolog 25:25
Xie JS, Donaldson L, Margolin EA (2023) Swelling of atrophic optic discs in idiopathic intracranial hypertension. J Neuroophthalmol 44(2):212–218
Srija YN, Pattnaik L, Mishra S, Panigrahi PK (2024) An optical coherence study on optic disc parameters and peripapillary retinal nerve fiber layer thickness in patients with optic disc edema. Indian J Ophthalmol 72:S96–S100
Jensen R, Skau M (2010) Disease activity in idiopathic intracranial hypertension: a 3-month follow-up study. J Headache Pain 1:S71
Scott CJ, Kardon RH, Lee AG, Frisen L, Wall M (2010) Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol 128:705–711
Skau M, Sander B, Milea D, Jensen R (2011) Disease activity in idiopathic intracranial hypertension: a 3-month follow-up study. J Neurol 258:277
Yri HM, Jensen RH (2015) Idiopathic intracranial hypertension: clinical nosography and field-testing of the ICHD diagnostic criteria. A case–control study. Cephalalgia 35:553–562
Auinger P, Durbin M, Feldon S, Garvin M, Kardon R, Keltner J, Kupersmith M, Sibony P, Plumb K, Wang JK, Werner JS (2014) Baseline OCT measurements in the idiopathic intracranial hypertension treatment trial, part I: quality control, comparisons, and variability. Invest Ophthalmol Vis Sci 55:8180–8188
Bingol Kiziltunc P, Atilla H (2021) A novel biomarker for increased intracranial pressure in idiopathic intracranial hypertension. Jpn J Ophthalmol 65(3):416–422
Attia R, Fitoussi R, Mairot K, Demortiere S, Stellman JP, Tilsley P, Audoin B, David T, Stolowy N (2023) Risk factors associated with progression from papilloedema to optic atrophy: results from a cohort of 113 patients. BMJ Open Ophthalmol 8:e001375
Wang JR, Linton EF, Johnson BA, Kupersmith MJ, Garvin MK, Kardon RH (2024) Visualization of optic nerve structural patterns in papilledema using deep learning variational autoencoders. Transl Vis Sci Technol 13:13
Monteiro ML, Afonso CL (2014) Macular thickness measurements with frequency domain-OCT for quantification of axonal loss in chronic papilledema from pseudotumor cerebri syndrome. Eye 28:390–398
Goldhagen BE, Bhatti MT, Srinivasan PP, Chiu SJ, Farsiu S, El-Dairi MA (2015) Retinal atrophy in eyes with resolved papilledema detected by optical coherence tomography. J Neuroophthalmol 35:122–126
Kabatas N, Eren Y, Nalcacioglu P, Caliskan S, Bicer T, Comoglu SS, Gurdal C (2021) Management of the regression of papilledema with regional axon loss in idiopathic intracranial hypertension patients. Int Ophthalmol 41:1467–1477
Rehman O, Ichhpujani P, Singla E, Negi R, Kumar S (2022) Change in contrast sensitivity and OCT parameters in idiopathic intracranial hypertension. Ther Adv Ophthalmol 14:25158414221083360
Chen JJ, Thurtell MJ, Longmuir RA, Garvin MK, Wang JK, Wall M, Kardon RH (2015) Causes and prognosis of visual acuity loss at the time of initial presentation in idiopathic intracranial hypertension. Invest Ophthalmol Vis Sci 56:3850–3859
Labib D, Abdel Raouf D (2015) Diagnostic value of optical coherence tomography in patients with idiopathic intracranial hypertension. Egypt J Neurol Psychiatry Neurosurg 52:249
Moss HE, Park JC, McAnany JJ (2015) The photopic negative response in idiopathic intracranial hypertension. Invest Ophthalmol Vis Sci 56:3709–3714
Park JC, Moss HE, McAnany JJ (2018) Electroretinography in idiopathic intracranial hypertension: comparison of the pattern ERG and the photopic negative response. Doc Ophthalmol 136:45–55
Chen Q, Feng C, Zhao G, Chen W, Wang M, Sun X, Sha Y, Li Z, Tian G (2020) Pseudotumour cerebri syndrome in China: a cohort study. Sci Rep 10:1222
Nogueira PF, Caiado GC, Gracitelli CPB, Martins FM, Barros FCD, Matas SLA, Teixeira SH, Noia LDC, Paulo DA (2021) Association between optical coherence tomography measurements and clinical parameters in idiopathic intracranial hypertension. J Ophthalmol 2021:1401609
Wibroe EA, Malmqvist L, Hamann S (2021) OCT based interpretation of the optic nerve head anatomy and prevalence of optic disc drusen in patients with idiopathic intracranial hypertension (IIH). Life (Basel) 11:584
Bassi ST, Pamu R, Ambika S, Praveen S, Priyadarshini D, Dharini V, Padmalakshmi K (2024) Optical coherence tomography in papilledema: a probe into the intracranial pressure correlation. Indian J Ophthalmol 62:1146–1151
Touzé R, Bonnin S, Houdart E, Nicholson P, Bodaghi B, Shotar E, Clarençon F, Lenck S, Touitou V (2021) Long-term kinetic papilledema improvement after venous sinus stenting in idiopathic intracranial hypertension. Clin Neuroradiol 31:483–490
Thaller M, Homer V, Mollan SP, Sinclair AJ (2023) Asymptomatic idiopathic intracranial hypertension: prevalence and prognosis. Clin Exp Ophthalmol 51:598–606
Albrecht P, Blasberg C, Ringelstein M, Müller AK, Finis D, Guthoff R, Kadas EM, Lagreze W, Aktas O, Hartung HP, Paul F, Brandt AU, Methner A (2017) Optical coherence tomography for the diagnosis and monitoring of idiopathic intracranial hypertension. J Neurol 264:1370–1380
Sheils CR, Fischer WS, Hollar RA, Blanchard LM, Feldon SE (2018) The relationship between optic disc volume, area, and Frisén score in patients with idiopathic intracranial hypertension. Am J Ophthalmol 195:101–109
Vijay V, Mollan SP, Mitchell JL, Bilton E, Alimajstorovic Z, Markey KA, Fong A, Walker JK, Lyons HS, Yiangou A, Tsermoulas G, Brock K, Sinclair AJ (2020) Using optical coherence tomography as a surrogate of measurements of intracranial pressure in idiopathic intracranial hypertension. JAMA Ophthalmol 138:1264–1271
Chang YC, Alperin N, Bagci AM, Lee SH, Rosa PR, Giovanni G, Lam BL (2015) Relationship between optic nerve protrusion measured by OCT and MRI and papilledema severity. Invest Ophthalmol Vis Sci 56:2297–2302
Eren Y, Kabatas N, Guven H, Comoglu S, Gurdal C (2019) Evaluation of optic nerve head changes with optic coherence tomography in patients with idiopathic intracranial hypertension. Acta Neurol Belg 119:351–357
Rehman O, Ichhpujani P, Kumar S, Saroa R, Sawal N (2022) Idiopathic intracranial hypertension and visual function in North Indian population. Eur J Ophthalmol 32(2):1186–1193
Kaya FS, Arici C (2023) Assessment of peripapillary choroidal thicknesses and optic disc diameters in idiopathic intracranial hypertension. Can J Ophthalmol 58:212–218
Sinclair AJ, Burdon MA, Nightingale PG, Ball AK, Good P, Matthews TD, Jacks A, Lawden M, Clarke CE, Stewart PM, Walker EA, Tomlinson JW, Rauz S (2010) Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ 341:c2701
Sibony PA, Kupersmith MJ, Feldon SE, Wang JK, Garvin M, Auinger P, Durbin M, Garvin MK, Kardon RH, Keltner J, Wang JK, Kupersmith M, Cello K, Werner JS (2015) Retinal and choroidal folds in papilledema. Invest Ophthalmol Vis Sci 56:5670–5680
Reggie SN, Avery RA, Bavinger JC, Jivraj I, Alfaro C, Pistilli M, Kohli AA, Liu GT, Shindler KS, Ross AG, Kardon RH, Sibony PA, Tamhankar M (2021) The sensitivity and specificity of retinal and choroidal folds to distinguish between mild papilloedema and pseudopapilledema. Eye (Basingstoke) 35(11):3131–3136
Gampa A, Vangipuram G, Shirazi Z, Moss HE (2017) Quantitative association between peripapillary Bruch’s membrane shape and intracranial pressure. Invest Ophthalmol Vis Sci 58:2739–2745
Banik R, Kupersmith MJ, Wang JK, Garvin MK (2019) The effect of acetazolamide and weight loss on intraocular pressure in idiopathic intracranial hypertension patients. J Glaucoma 28:352–356
Panyala R, Sharma P, Sihota R, Saxena R, Prasad K, Phuljhele S, Gurrala S, Bhaskaran K (2021) Role of spectral domain optical coherence tomography in the diagnosis and prognosis of papilledema. Indian J Ophthalmol 69:2372–2377
Pasaoglu I, Satana B, Altan C, Artunay O, Basarir B, Onmez FE, Inal A (2019) Lamina cribrosa surface position in idiopathic intracranial hypertension with swept-source optical coherence tomography. Indian J Ophthalmol 67:1085–1088
Tatar IT, Solmaz B, Erdem ZG, Pasaoglu I, Demircan A, Tülü Aygün B, Ozkaya A (2020) Morphological assessment of lamina cribrosa in idiopathic intracranial hypertension. Indian J Ophthalmol 68:164–167
Ozdemir I, Çevik S (2020) Measurement of choroid thickness using optical coherence tomography to monitor intracranial pressure in an idiopathic cranial hypertension model. Neurol India 68:636–639
Pahuja A, Dhiman R, Aggarwal V, Aalok SP, Saxena R (2024) Evaluation of peripapillary and macular optical coherence tomography angiography characteristics in different stages of papilledema. J Neuroophthalmol 44:53–60
Dreesbach M, Joachimsen L, Kuchlin S, Reich M, Gross NJ, Brandt AU, Schuchardt F, Harloff A, Bohringer D, Lagreze WA (2020) Optic nerve head volumetry by optical coherence tomography in papilledema related to idiopathic intracranial hypertension. Transl Vis Technol 9:24
Lin Y, Chen S, Zhang M (2021) Peripapillary vessel density measurement of quadrant and clock-hour sectors in primary angle closure glaucoma using optical coherence tomography angiography. BMC Ophthalmol 21:328
Mansoori T, Balakrishna N (2019) Peripapillary vessel density and retinal nerve fiber layer thickness in patients with unilateral primary angle closure glaucoma with superior hemifield defect. J Curr Glaucoma Pract 13:21–27
Borrelli E, Parravano M, Sacconi R, Costanzo E, Querques L, Vella G, Bandello F, Querques G (2020) Guidelines on optical coherence tomography angiography imaging: 2020 focused update. Ophthalmol Ther 9:697–707
Tüntaş Bilen F, Atilla H (2019) Peripapillary vessel density measured by optical coherence tomography angiography in idiopathic intracranial hypertension. J Neuroophthalmol 39:319–323
Yalcinkaya Cakir G, Solmaz B, Cakir I, Pasaoglu IB, Taskapili M (2024) Optical coherence tomography angiography findings in optic disc drusen and idiopathic intracranial hypertension. Eur J Ophthalmol 34:566–573
Kaya FS, Sonbahar O, Açar PA, Özbaş M, Yigit FU (2021) Evaulating peripapillary vessel density ın regressed papilledema ın ıdiopathic ıntracranial hypertension patients. Photodiagn Photodyn Ther 36:102551
Fard MA, Sahraiyan A, Jalili J, Hejazi M, Suwan Y, Ritch R, Subramanian PS (2019) Optical coherence tomography angiography in papilledema compared with pseudopapilledema. Invest Ophthalmol Vis Sci 60:168–175
Wang H, Cao L, Kwapong WR, Liu G, Wang R, Liu J, Wu B (2023) Optic nerve head changes measured by swept source optical coherence tomography and angiography in patients with intracranial hypertension. Ophthalmol Ther 12:3295–3305
Chonsui M, Le Goff M, Korobelnik JF, Rougier MB (2022) Quantitative analysis of radial peripapillary capillary network in patients with papilledema compared with healthy subjects using optical coherence tomography angiography. J Neuroophthalmol 42:e109–e115
Kwapong WR, Cao L, Pan R, Wang H, Ye C, Tao W, Liu J, Wu B (2023) Retinal microvascular and structural changes in intracranial hypertension patients correlate with intracranial pressure. CNS Neurosci Ther 29:4093–4101
Adhi M, Duker JS (2013) Optical coherence tomography—current and future applications. Curr Opin Ophthalmol 24:213–221
Gabriele ML, Wollstein G, Ishikawa H, Kagemann L, Xu J, Folio LS, Schuman JS (2011) Optical coherence tomography: history, current status, and laboratory work. Invest Ophthalmol Vis Sci 52:2425–2436
Ratchford JN, Quigg ME, Conger A, Frohman T, Frohman E, Balcer LJ, Calabresi PA, Kerr DA (2009) Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology 73:302–308
Stricker S, Oberwahrenbrock T, Zimmermann H, Schroeter J, Endres M, Brandt AU, Paul F (2011) Temporal retinal nerve fiber loss in patients with spinocerebellar ataxia type 1. PLoS ONE 6:e23024
Moschos MM, Tagaris G, Markopoulos I, Margetis I, Tsapakis S, Kanakis M, Koutsandrea C (2011) Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur J Ophthalmol 21:24–29
Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, Calabresi PA, Polman C (2010) Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 9:921–932
Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT (2005) The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res 24:39–73
Bellezza AJ, Hart RT, Burgoyne CF (2000) The optic nerve head as a biomechanical structure: initial finite element modeling. Invest Ophthalmol Vis Sci 41:2991–3000
Strouthidis NG, Fortune B, Yang H, Sigal IA, Burgoyne CF (2011) Effect of acute intraocular pressure elevation on the monkey optic nerve head as detected by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 52:9431–9437
Liu Z, Dong C, Wang X, Han X, Zhao P, Lv H, Li Q, Wang Z (2015) Association between idiopathic intracranial hypertension and sigmoid sinus dehiscence/diverticulum with pulsatile tinnitus: a retrospective imaging study. Neuroradiology 57:747–753
Prabhat N, Kaur K, Takkar A, Ahuja C, Katoch D, Goyal M, Dutta P, Bhansali A, Lal V (2024) Pituitary dysfunction in idiopathic intracranial hypertension: an analysis of 80 patients. Can J Neurol Sci 51:265–271
Falsini B, Tamburrelli C, Porciatti V, Anile C, Porrello G, Mangiola N (1992) Pattern electroretinograms and visual evoked potentials in idiopathic intracranial hypertension. Ophthalmologica 205:194–203
Madill SA, Connor SE (2005) Computed tomography demonstrates short axial globe length in cases with idiopathic intracranial hypertension. J Neuroophthalmol 25:180–184
Yadav SK, Kadas EM, Motamedi S, Polthier K, Haußer F, Gawlik K, Paul F, Brandt A (2018) Optic nerve head three-dimensional shape analysis. J Biomed Opt 23:1–13
Mittra RA, Sergott RC, Flaharty PM, Lieb WE, Savino PJ, Bosley TM, Hedges TR Jr (1993) Optic nerve decompression improves hemodynamic parameters in papilledema. Ophthalmology 100:987–997
Hayreh SS, Zimmerman MB (2008) Non-arteritic anterior ischemic optic neuropathy: role of systemic corticosteroid therapy. Graefes Arch Clin Exp Ophthalmol 246:1029–1046
Sharma S, Ang M, Najjar RP, Sng C, Cheung CY, Rukmini AV, Schmetterer L, Milea D (2017) Optical coherence tomography angiography in acute non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol 101:1045–1051
Mohammadi S, Gouravani M, Salehi MA, Arevalo JF, Galetta SL, Harandi H, Frohman EM, Frohman TC, Saidha S, Sattarnezhad N, Paul F (2023) Optical coherence tomography angiography measurements in multiple sclerosis: a systematic review and meta-analysis. J Neuroinflamm 20:85
Borrelli E, Balasubramanian S, Triolo G, Barboni P, Sadda SR, Sadun AA (2018) Topographic macular microvascular changes and correlation with visual loss in chronic leber hereditary optic neuropathy. Am J Ophthalmol 192:217–228
Malhotra K, Padungkiatsagul T, Moss HE (2020) Optical coherence tomography use in idiopathic intracranial hypertension. Ann Eye Sci 5:7
Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G (2018) Optical coherence tomography angiography. Prog Retin Eye Res 64:1–55
Querfurth HW, Lagrèze WD, Hedges TR, Heggerick PA (2002) Flow velocity and pulsatility of the ocular circulation in chronic intracranial hypertension. Acta Neurol Scand 105:431–440
Skau M, Milea D, Sander B, Wegener M, Jensen R (2011) OCT for optic disc evaluation in idiopathic intracranial hypertension. Graefes Arch Clin Exp Ophthalmol 249:723–730
Afonso CL, Raza AS, Kreuz AC, Hokazono K, Cunha LP, Oyamada MK, Monteiro ML (2015) Relationship between pattern electroretinogram, frequency-domain OCT, and automated perimetry in chronic papilledema from pseudotumor cerebri syndrome. Invest Ophthalmol Vis Sci 56:3656–3665
Banerjee M, Phuljhele S, Saluja G, Kumar P, Saxena R, Sharma P, Vibha D, Pandit AK (2022) Optical coherence tomography features and correlation of functional and structural parameters in patients of idiopathic intracranial hypertension. Indian J Ophthalmol 70:1343–1349
Thaller M, Homer V, Hyder Y, Yiangou A, Liczkowski A, Fong AW, Virdee J, Piccus R, Roque M, Mollan SP, Sinclair AJ (2023) The idiopathic intracranial hypertension prospective cohort study: evaluation of prognostic factors and outcomes. J Neurol 270:851–863
Rodriguez Torres Y, Lee P, Mihlstin M, Tomsak RL (2021) Correlation between optic disc peripapillary capillary network and papilledema grading in patients with idiopathic intracranial hypertension: a study of optical coherence tomography angiography. J Neuroophthalmol 41:48–53
El-Haddad N, Ismael SA, El-Wahab AA, Shalaby S, Farag MMA, Mohammd NS, Shawky S (2023) Optic disc vessel density changes after shunt surgery in idiopathic intracranial hypertension. Photodiagn Photodyn Ther 42:103625
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was not funded by any specific funding agencies.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design and conceptualization. Mallika Prem Senthil and Jose Estevez Bordon conducted the literature search. Mallika Prem Senthil, Ranjay Chakraborty, Paul Constable, Shannon Brown, and Simu Simon did the data curation and analysis. The risk of bias assessment was done by Saumya Anand and Dalia Al-Dasooqi. The initial manuscript draft was written by Mallika Prem Senthil with input from all authors on previous versions. All authors reviewed and endorsed the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
No potential conflict of interest was reported by the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prem Senthil, M., Anand, S., Chakraborty, R. et al. Exploring the utility of retinal optical coherence tomography as a biomarker for idiopathic intracranial hypertension: a systematic review. J Neurol (2024). https://doi.org/10.1007/s00415-024-12481-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12481-3