Abstract
Background
Postural imbalance and falls are an early disabling symptom in patients with progressive supranuclear palsy (PSP) of multifactorial origin that may involve abnormal vestibulospinal reflexes. Low-intensity noisy galvanic vestibular stimulation (nGVS) is a non-invasive treatment to normalize deficient vestibular function and attenuate imbalance in Parkinson’s disease. The presumed therapeutic mode of nGVS is stochastic resonance (SR), a mechanism by which weak sensory noise stimulation can enhance sensory information processing.
Objective
To examine potential treatment effects of nGVS on postural instability in 16 patients with PSP with a clinically probable and [18F]PI-2620 tau-PET-positive PSP.
Methods
Effects of nGVS of varying intensity (0–0.7 mA) on body sway were examined, while patients were standing with eyes closed on a posturographic force plate. We assumed a bell-shaped response curve with maximal sway reductions at intermediate nGVS intensities to be indicative of SR. An established SR-curve model was fitted on individual patient outcomes and three experienced human raters had to judge whether responses to nGVS were consistent with the exhibition of SR.
Results
We found nGVS-induced reductions of body sway compatible with SR in 9 patients (56%) with optimal improvements of 31 ± 10%. In eight patients (50%), nGVS-induced sway reductions exceeded the minimal clinically important difference (improvement: 34 ± 5%), indicative of strong SR.
Conclusion
nGVS yielded clinically relevant reductions in body sway compatible with the exhibition of SR in vestibular sensorimotor pathways in at least half of the assessed patients. Non-invasive vestibular noise stimulation may be thus a well-tolerated treatment strategy to ameliorate postural symptoms in PSP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progressive supranuclear palsy (PSP) is a rare and rapidly progressing neurodegenerative disease that clinically belongs to the group of atypical parkinsonian syndromes and is characterized by cerebral aggregation of tau protein [7, 53]. Early postural instability and unexplained recurrent falls are central to the clinical presentation of PSP and a diagnostic criterium to differentiate the disease from idiopathic Parkinson’s disease [24, 35]. Postural symptoms are a major disabling factor of PSP and a determinant of survival due to the associated risk of secondary injuries and immobilization [9, 42]. Balance deficits in PSP are likely of multifactorial origin and may involve axial rigidity, supranuclear gaze palsy, and deficient central sensorimotor balance regulation including abnormal vestibular balance reflexes [9, 36, 69]. As of yet, there is no disease-modifying therapy available for PSP, and existing medical and non-medical approaches to improve balance and prevent falls in patients yield moderate and transient improvements, if any [7, 9, 11, 51, 52].
A vestibular origin of postural symptoms and falls in PSP has been repeatedly discussed [5, 36, 56, 69]. There is, albeit conflicting, clinical evidence for abnormal peripheral vestibular function related to otolith pathways in PSP [5, 21, 36, 41, 56]. Vestibular deficits could be an accompanying age-related symptom, as PSP is a late-onset disease, or disease-specific directly associated with the pathophysiology of PSP. Brain imaging studies in patients with Parkinson’s disease and PSP further point to a common dysfunction within central cholinergic networks that transmit vestibular afferent inputs to the thalamus and basal ganglia, which closely correlates with postural deficits and falls in afflicted patients [6, 40, 69]. A key node within this network is the pedunculopontine nucleus (PPN) that provides excitatory cholinergic input to the thalamus and undergoes substantial neuronal loss early in the course of PSP [68]. Previous attempts using deep brain stimulation in PSP have already identified the PPN as a potential therapeutic target, however, without yielding clear and consistent benefit in tested patients [47]. A non-invasive method to modulate PPN activity is galvanic vestibular stimulation (GVS) [10]—a technique to activate vestibular afferents by weak electric current [13]. Delivered as a low-intensity and imperceptible random noise stimulus (called noisy GVS; nGVS), it has been previously shown to improve postural imbalance and a range of motor and non-motor symptoms in patients with Parkinson’s disease [34, 43, 44, 46, 55, 66, 67]. The presumed therapeutic mode underlying treatment effects of nGVS is stochastic resonance (SR)—a mechanism by which sensory information processing becomes enhanced at the presence of a particular non-zero amount of sensory noise [12, 37, 62].
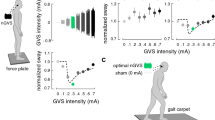
The aim of this study was to examine potential treatment effects of vestibular neuromodulation via nGVS on postural instability in patients with PSP. It is known from previous studies that treatment effects of nGVS critically depend on the stimulation intensity. Intermediate noise intensities improve vestibular signal transfer whereas low or high stimulation intensities either not affect or even disturb signal processing [18, 38]. Accordingly, the characteristic signature indicating nGVS-induced SR and a positive treatment response is a bell-shaped performance curve, where the performance metric (i.e., postural balance) becomes optimally enhanced at a specific intermediate level of noise (Fig. 1). To identify nGVS-induced balance improvements in patients with PSP, we therefore studied postural responses to nGVS across a broad range of stimulation intensities and used different established criteria to determine whether stimulation-induced modulations of postural instability are compatible with the exhibition of SR. We further studied whether demographic or disease-related characteristics (e.g., duration of disease, severity of symptoms, or tau-deposition pattern) are associated with potential treatment responses.
Experimental setup and procedures. Left side: Effects of noisy galvanic vestibular stimulation (nGVS) on static balance in patients were measured on a posturographic force plate. Velocity of body sway was calculated from the resultant center-of-pressure trajectories. Right side: Exemplary modulation of body sway (hypothetical data, lower panel) across the administered nGVS intensities (upper panel) that follows a bell-shaped performance curve indicative of the presence of stochastic resonance (model fit: dashed line). Filled dots indicate body sway reductions greater than the minimally important difference (grey area). The green filled dot indicates the optimal reduction of body sway at a particular nGVS level
Materials and methods
Participants
Sixteen patients (age 70.7 ± 7.8 years, 6 females) with a probable PSP according to the MDS-PSP criteria [24] participated in the study (for detailed patient characteristics, see Table 1). [18F]PI-2620 PET imaging was performed to depict tau-deposits as an in vivo biomarker supporting the clinical diagnosis [8]. Each patient underwent a complete physical, neurological and neuro-otological examination by an expert neurologist, including a clinical assessment of vestibuloocular (head impulse test) and vestibulospinal (Romberg’s test) function (AZ). Symptom severity was scored using the Progressive Supranuclear Palsy Rating Scale (PSPRS) [20], while patients were taking their regular medication (i.e., L-dopa at a mean daily dose of 518 ± 254 mg in 10 patients). Patients had a mild-to-moderate disease severity with a PSPRS of 29.8 ± 11.8. Fourteen age-matched healthy controls (age 68.9 ± 6.5 years, 9 females) were included in the study to establish normative data. All participants gave written informed consent prior to study inclusion.
PET imaging and data analysis
[18F]PI-2620 PET imaging was performed in a full dynamic setting (0–60 min post injection) with a Siemens Biograph or mCT PET/CT scanner (Siemens Healthineers, Erlangen, Germany) at the Department of Nuclear Medicine, LMU Munich as described earlier [8, 57]. The multilinear reference tissue model 2 (MRTM2) in PMOD version 3.9 (PMOD Inc) was used to calculate distribution volume ratio images (DVR; DVR = non-displaceable binding potential + 1) of each full dynamic data set. The cerebellum excluding the dentate nucleus and the central cerebellar white matter as well as the superior and the posterior cerebellar layers (thickness in z direction = 1.5 cm each) served as a reference region.
Two expert readers (M.B. and M.Z.) performed a dichotomous visual read of DVR maps. In addition, [18F]PI-2620 DVR values were obtained in the following PSP target regions predefined by Brainnetome atlas [14]: frontal lobe, thalamus, globus pallidus, and putamen (left- and right-sided, respectively). Patient data were compared to a normative in-house data set derived from a matched healthy control group of the same scanners.
Galvanic vestibular stimulation
Vestibular noise stimulation (i.e., nGVS) was applied via a pair of 4.0 cm × 6.0 cm Ag–AgCl electrodes attached bilaterally over the left and right mastoid process. Zero-mean Gaussian white noise stimulation with a frequency range of 0–30 Hz and varying peak amplitudes of 0–0.7 mA was delivered by a mobile constant current stimulator (neuroConn®, Illmenau, Germany).
Experimental procedures
Body sway was recorded for 30 s on a posturographic force plate (Kistler, 9261A, Kistler Group, Winterthur, Switzerland) at 40 Hz while patients were standing with their eyes closed (Fig. 1A). This procedure was repeated eight times, while patients were stimulated with a different amplitude of nGVS (ranging from 0 to 0.7 mA, in a randomized order) in each trial. Patients were blinded to the exact stimulation order. Between trials, patients were given a short break to recover.
Data and statistical analysis
For each stance trial, mean sway velocity was calculated as the primary output measures based on the recorded radial center-of-pressure (CoP) trajectory using the formula \(SV = 1/T \times {\sum }_{i}\left|{r}_{i+1}-{r}_{i}\right|, [{\text{mm}}/{\text{s}}]\), where \(T\) is the total trial duration (i.e., 30 s) and \({r}_{i}\) is the radial CoP distance of the ith sample. For further analysis, sway velocity measures from the eight stance trials were normalized to sway velocity obtained during 0 mA stimulation (i.e., baseline condition).
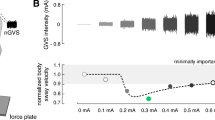
To determine whether SR-like dynamics were present in the balance responses of patients to varying nGVS levels, we tested three increasingly rigorous criteria that built on one another: (1) The first criterion tested whether body sway of patients improved for at least one particular nGVS level compared to baseline condition (i.e., 0 mA nGVS). (2) The second criterion was based on a visual inspection of response dynamics of body sway across increasing nGVS level by three experienced human raters (i.e., MW, DP, and AZ). Each rater had to evaluate whether (in addition to the fulfillment of the first criterion) nGVS-amplitude-dependent changes of body sway in individual patients were further compatible with a bell-shaped response curve with improvements of performance at intermediate stimulation intensities that is indicative of the presence of SR. This evaluation was based on a plot of the normalized nGVS-dependent changes in body sway and a concomitant plot of a theoretical SR curve that was fit on the data using a goodness-of-fit statistic [2, 18] (Fig. 1B). The applied equation fit represents an adapted version of the originally proposed SR model by Benzi [3], including a piecewise, linear masking effect to model cases where nGVS effects at high amplitudes may have detrimental effects on the performance metric [58]. The criterion was met if at least two of the raters identified the presence of SR-like dynamics. (3) The third criterion additionally evaluated whether improvements at intermediate nGVS levels were greater than the minimal clinically important difference (MCID; defined as half the standard deviation for normative data [60]) for changes in body sway velocity. MCID for sway velocity was 2.3 mm/s calculated based on the posturographic recordings of the 14 age-matched healthy individuals standing with eyes closed for 30 s.
Based on the three criteria, patients were classified as showing solely optimal improvement and no SR (criterion 1), exhibiting weak SR (criterion 1 & 2) or showing strong SR (criterion 1 & 2 & 3). Potential correlations between SR classification and age, gender, disease duration, disease severity (i.e., PSPRS), and baseline body sway were analyzed using Spearman's rank correlation. Extent of Tau deposition in predefined regions of interest was compared between patients with weak/strong SR and no SR using multivariate analysis of variance including age and sex as covariates and Bonferroni adjustment for multiple comparisons. Descriptive statistics are reported as mean ± SD. All results were considered significant at p < 0.05. Statistical analysis was performed using SPSS (Version 26.0, IBM Corp., USA).
Results
Application of nGVS at intensities ranging from 0.1 to 0.7 mA was well tolerated and did not cause apparent disequilibrium in any of the examined patients. In a first step of analysis, we evaluated whether body sway velocity was decreased by at least one particular nGVS intensity compared to sham stimulation (i.e., nGVS at 0 mA). This criterion was met by 12 patients (75%) with an optimal mean improvement magnitude of 25% (range 2–45%) at an average intensity of 0.4 mA (range 0.1–0.7 mA).
In a second step, an established SR model was fit to the individual modulations of body sway velocity in dependence of nGVS intensity (Fig. 2). Three experts were asked to independently rate for each patient by visual inspection of individual sway velocity modulations and corresponding model fits, whether body sway responses follow a bell-shaped performance curve or not. Based on their judgments, SR-like treatment responses to nGVS were present in nine patients (56%) with optimal improvements of 31% (range 7–45%) at an average intensity of 0.3 mA (range 0.1–0.5 mA). Analogous bell-shaped performance modulations with optimal improvement at intermediate noise intensities were found on the group average response level of these patients (Fig. 3). In the remaining patients (44%), body sway velocity either randomly fluctuated (3 patients) or was generally increased (4 patients) across the range of tested nGVS intensities.
Individual effects of low-intensity vestibular noise stimulation on static balance. Normalized body sway responses to noisy galvanic vestibular stimulation (nGVS) are plotted against the administered nGVS levels for each individual patient. Dashed lines represent the stochastic resonance (SR) model fits. Black filled dots indicate body sway modulations greater than the minimally important clinical difference (grey area). Green filled dots indicate maximum reductions of body sway at particular nGVS levels. Blue asterisks denote those patients that exhibit SR-like responses according to three human judges (weak SR). Pink crosses denote those patients that additionally show clinically meaningful improvement of body sway (strong SR)
Group average effects of low-intensity vestibular noise stimulation on static balance. Group average normalized body sway responses (mean ± SEM) to noisy galvanic vestibular stimulation (nGVS) are plotted for each of the administered nGVS levels for all patients (left panel), those patients exhibiting at least weak stochastic resonance (SR; middle panel), and those exhibiting strong SR (right panel). Filled dots indicate body sway modulations greater than the minimally important clinical difference (grey area)
We subsequently identified those patients that in addition to SR-like response dynamics showed a clinically meaningful improvement of static balance (i.e., a reduction of body sway velocity greater than the MCID, Fig. 2). This criterion for the exhibition of strong SR was met by 8 patients (50%) with a mean optimal improvement of 34% (range 28–45%) at an average intensity of 0.3 mA (range 0.1–0.5 mA). Considerable SR-like performance improvements were also apparent on the group average level of patients exhibiting strong SR (Fig. 3).
In a final step, we explored demographic and disease-related factors that may potentially promote or hamper the exhibition of weak or strong SR in response to nGVS treatment. We did not find any correlation between age, gender, disease duration, PSPRS, or body sway at baseline and nGVS treatment responses of individual patients. In addition, mean DVR in the left and right frontal lobe, thalamus, putamen, and globus pallidus were not statistically different for patients with and without SR. Expectedly, whole-brain DVR and mean DVR in the bilateral thalamus, putamen, and globus pallidus were significantly higher in both PSP subgroups compared to the control group (Fig. 4).
[18F]PI-2620 binding in PSP subgroups and controls. A Average [18F]PI-2620 distribution volume ratio (DVR) maps presented as axial overlays on a standard magnetic resonance imaging template for all study groups. Extracerebral voxels were masked. B [18F]PI-2620 DVR comparisons for selected progressive supranuclear palsy (PSP) target regions between healthy controls and patients that respond or do not respond to treatment with noisy galvanic vestibular stimulation (nGVS)
Discussion
In this study, we examined the effects of a weak vestibular neuromodulation by nGVS on postural instability in patients with PSP. The purported mode of action of nGVS is SR, by which sensory-(motor) processing can become enhanced at the presence of low-intensity additive sensory noise below the sensory detection threshold [12, 37, 62]. In half of the assessed patients with PSP, we found robust stimulation responses compatible with SR that were linked to clinically meaningful improvements in static balance regulation, i.e., 28–45% reduction in sway velocity—a measure that has been closely linked to the frequency of falls in afflicted patients [69]. The observed response rate to treatment is higher compared to healthy individuals, where nGVS-induced balance responses compatible with SR were only rarely reported [2]. Consistent with previous reports [18, 66], the average nGVS intensity that optimally improved postural sway was found at 0.3 mA, which corresponds to approximately 60% of the estimated detection threshold of vestibular afferent responses to GVS [33]. Previous studies provide accumulating evidence that nGVS can attenuate postural imbalance and other motor and autonomic symptoms in patients with Parkinson’s disease [34, 43, 44, 46, 55, 66, 67]. To our knowledge, this is the first study suggesting that nGVS treatment effects may extend to postural symptoms in patients with PSP.
Multifaceted reasons for postural imbalance and falls in PSP are currently discussed that point to at least two distinct mechanisms of action by which nGVS could impact postural symptoms in afflicted patients. First, there is, however not uncontroversial, evidence that links disequilibrium and falls in PSP to vestibular dysfunction [5, 21, 36, 41, 56]. Accordingly, the previous reports suggest that in particular otolith-mediated ocular-motor and balance reflexes can be impaired in patients with PSP [5, 36, 56]. In this context, nGVS-induced facilitation of vestibular signal processing has been shown to not only sensitize vestibular perception as such [18, 31, 32, 63], but to also enhance the responsiveness of vestibulospinal balance reflexes in healthy individuals and patients with chronic vestibular hypofunction [48, 61]. Improved vestibulospinal function by nGVS has further been associated with a stabilization of balance during standing and walking under vestibular-challenging circumstances in both cohorts [15, 17, 19, 25,26,27,28, 39, 64, 65]. Hence, nGVS could attenuate postural imbalance in PSP by restoring deficient vestibular-related balance responses in afflicted patients.
Alternatively, nGVS treatment in PSP could take effect along ascending pathways that connect vestibular afferents to the thalamus and basal ganglia [10, 54]. The PPN, a central neuronal hub along this pathway, provides the primary cholinergic input to the thalamus. Natural vestibular input as well as vestibular neuromodulation via nGVS were shown to directly modulate PPN activity in animal models and humans [1, 10, 49]. In PSP, neuronal loss in the PPN and the thalamus is a common and early neurodegenerative sign in the course of disease [22, 29, 59, 68], which entails a substantial reduction of thalamic cholinergic activity in afflicted patients [23]. PSP-associated neurodegeneration within this mesencephalic brainstem–thalamus loop appears to be further closely associated with patients’ postural imbalance and frequency of falling as suggested by functional brain imaging [9, 69]. Hence, nGVS may attenuate postural instability in PSP via an activation of tegmental brainstem nuclei that restores excitatory cholinergic input to the thalamus.
It is eventually also conceivable that nGVS treatment effects on posture in PSP may be rather unrelated to the primary PSP pathophysiology. The average age of disease onset in PSP is in the sixth decade of life [45] corresponding to mean disease onset at 67 years (range 49–77 years) in our cohort of patients. Vestibular sensitive capacity and related motor functions are known to gradually decline above the age of 40 [4]. A close association between postural imbalance and age-related decline in vestibular–perceptual thresholds has been previously reported [30]. This may explain why beneficial responses of vestibular perceptual and sensorimotor function to nGVS treatment become more frequent and pronounced in the elderly compared to the young healthy population [16, 25, 50]. Age rather than disease-related impairments in vestibular balance control could therefore also be the source of nGVS-induced balance improvements in patients with PSP. The latter assumption is supported by the lack of correlation between treatment responses and disease duration, disease severity scores, or the extent of tau load in PSP target regions derived from [18F]PI-2620 PET.
In summary, we found that vestibular neuromodulation via nGVS yielded clinically meaningful reductions of postural instability in half of the assessed patients with PSP. Long-term application of nGVS in patients has previously shown to be safe with negligible side effects. Non-invasive vestibular noise stimulation in PSP may therefore be a well-tolerated treatment option to attenuate postural symptoms, reduce the risk of falling, and preserve mobility in afflicted patients. A re-evaluation of the observed effects in a larger sample of patients is required to clarify whether and how the treatment response may depend on clinical characteristics of patients, including instrumented assessment of vestibular semicircular canal and otolith function, and whether long-term treatment in respondent patients can effectively facilitate mobility and reduce their risk of falling.
Data availability
The datasets used and/or analyzed during the current study will be available from the corresponding author upon reasonable request.
References
Aravamuthan BR, Angelaki DE (2012) Vestibular responses in the macaque pedunculopontine nucleus and central mesencephalic reticular formation. Neuroscience 223:183–199
Asslander L, Giboin LS, Gruber M, Schniepp R, Wuehr M (2021) No evidence for stochastic resonance effects on standing balance when applying noisy galvanic vestibular stimulation in young healthy adults. Sci Rep 11:12327
Benzi R, Sutera A, Vulpiani A (1981) The mechanism of stochastic resonance. J Phys A: Math Gen Appl Entomol 14:L453
Bermúdez Rey MC, Clark TK, Wang W, Leeder T, Bian Y, Merfeld DM (2016) Vestibular perceptual thresholds increase above the age of 40. Front Neurol 7:162–162
Bisdorff AR, Bronstein AM, Wolsley C, Lees AJ (1997) Torticollis due to disinhibition of the vestibulo-collic reflex in a patient with Steele–Richardson–Olszewski syndrome. Mov Disord 12:328–336
Bohnen NI, Müller ML, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, Albin RL (2009) History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology 73:1670–1676
Boxer AL, Yu JT, Golbe LI, Litvan I, Lang AE, Hoglinger GU (2017) Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol 16:552–563
Brendel M, Barthel H, van Eimeren T, Marek K, Beyer L, Song M, Palleis C, Gehmeyr M, Fietzek U, Respondek G, Sauerbeck J, Nitschmann A, Zach C, Hammes J, Barbe MT, Onur O, Jessen F, Saur D, Schroeter ML, Rumpf JJ, Rullmann M, Schildan A, Patt M, Neumaier B, Barret O, Madonia J, Russell DS, Stephens A, Roeber S, Herms J, Botzel K, Classen J, Bartenstein P, Villemagne V, Levin J, Hoglinger GU, Drzezga A, Seibyl J, Sabri O (2020) Assessment of 18F-PI-2620 as a biomarker in progressive supranuclear palsy. JAMA Neurol 77:1408–1419
Brown FS, Rowe JB, Passamonti L, Rittman T (2020) Falls in progressive supranuclear palsy. Mov Disord Clin Pract 7:16–24
Cai J, Lee S, Ba F, Garg S, Kim LJ, Liu A, Kim D, Wang ZJ, McKeown MJ (2018) Galvanic vestibular stimulation (GVS) augments deficient pedunculopontine nucleus (PPN) connectivity in mild Parkinson’s disease: fMRI effects of different stimuli. Front Neurosci 12:101
Clerici I, Ferrazzoli D, Maestri R, Bossio F, Zivi I, Canesi M, Pezzoli G, Frazzitta G (2017) Rehabilitation in progressive supranuclear palsy: effectiveness of two multidisciplinary treatments. PLoS ONE 12:e0170927
Collins J, Chow CC, Imhoff TT (1995) Stochastic resonance without tuning. Nature 376:236–238
Dlugaiczyk J, Wuehr M, Straka H (2020) Electrical stimulation of vestibular endorgans. In: Fritzsch B (ed) The senses: a comprehensive reference, 2nd edn. Elsevier, Oxford, pp 635–671
Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, Fox PT, Eickhoff SB, Yu C, Jiang T (2016) The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex 26:3508–3526
Fujimoto C, Egami N, Kawahara T, Uemura Y, Yamamoto Y, Yamasoba T, Iwasaki S (2018) Noisy galvanic vestibular stimulation sustainably improves posture in bilateral vestibulopathy. Front Neurol 9:900
Fujimoto C, Kinoshita M, Kamogashira T, Egami N, Kawahara T, Uemura Y, Yamamoto Y, Yamasoba T, Iwasaki S (2019) Noisy galvanic vestibular stimulation has a greater ameliorating effect on posture in unstable subjects: a feasibility study. Sci Rep 9:17189
Fujimoto C, Yamamoto Y, Kamogashira T, Kinoshita M, Egami N, Uemura Y, Togo F, Yamasoba T, Iwasaki S (2016) Noisy galvanic vestibular stimulation induces a sustained improvement in body balance in elderly adults. Sci Rep 6:37575
Galvan-Garza RC, Clark TK, Mulavara AP, Oman CM (2018) Exhibition of stochastic resonance in vestibular tilt motion perception. Brain Stimul 11:716–722
Goel R, Kofman I, Jeevarajan J, De Dios Y, Cohen HS, Bloomberg JJ, Mulavara AP (2015) Using low levels of stochastic vestibular stimulation to improve balance function. PLoS ONE 10:e0136335
Golbe LI, Ohman-Strickland PA (2007) A clinical rating scale for progressive supranuclear palsy. Brain 130:1552–1565
Goldschagg N, Bremova-Ertl T, Bardins S, Dinca N, Feil K, Krafczyk S, Lorenzl S, Strupp M (2019) No evidence of a contribution of the vestibular system to frequent falls in progressive supranuclear palsy. J Clin Neurol 15:339–346
Henderson JM, Carpenter K, Cartwright H, Halliday GM (2000) Loss of thalamic intralaminar nuclei in progressive supranuclear palsy and Parkinson’s disease: clinical and therapeutic implications. Brain 123(Pt 7):1410–1421
Hirano S, Shinotoh H, Shimada H, Aotsuka A, Tanaka N, Ota T, Sato K, Ito H, Kuwabara S, Fukushi K, Irie T, Suhara T (2010) Cholinergic imaging in corticobasal syndrome, progressive supranuclear palsy and frontotemporal dementia. Brain 133:2058–2068
Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I, Movement Disorder Society-endorsed PSPSG (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32:853–864
Inukai Y, Masaki M, Otsuru N, Saito K, Miyaguchi S, Kojima S, Onishi H (2018) Effect of noisy galvanic vestibular stimulation in community-dwelling elderly people: a randomised controlled trial. J Neuroeng Rehabil 15:63–63
Inukai Y, Otsuru N, Masaki M, Saito K, Miyaguchi S, Kojima S, Onishi H (2018) Effect of noisy galvanic vestibular stimulation on center of pressure sway of static standing posture. Brain Stimul 11:85–93
Iwasaki S, Fujimoto C, Egami N, Kinoshita M, Togo F, Yamamoto Y, Yamasoba T (2018) Noisy vestibular stimulation increases gait speed in normals and in bilateral vestibulopathy. Brain Stimul 11:709–715
Iwasaki S, Yamamoto Y, Togo F, Kinoshita M, Yoshifuji Y, Fujimoto C, Yamasoba T (2014) Noisy vestibular stimulation improves body balance in bilateral vestibulopathy. Neurology 82:969–975
Jellinger K (1988) The pedunculopontine nucleus in Parkinson’s disease, progressive supranuclear palsy and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 51:540–543
Karmali F, Bermudez Rey MC, Clark TK, Wang W, Merfeld DM (2017) Multivariate analyses of balance test performance, vestibular thresholds, and age. Front Neurol 8:578
Keywan A, Jahn K, Wuehr M (2019) Noisy galvanic vestibular stimulation primarily affects otolith-mediated motion perception. Neuroscience 399:161–166
Keywan A, Wuehr M, Pradhan C, Jahn K (2018) Noisy galvanic stimulation improves roll-tilt vestibular perception in healthy subjects. Front Neurol. https://doi.org/10.3389/fneur.2018.00083
Kwan A, Forbes PA, Mitchell DE, Blouin J-S, Cullen KE (2019) Neural substrates, dynamics and thresholds of galvanic vestibular stimulation in the behaving primate. Nat Commun 10:1904
Lee S, Smith PF, Lee WH, McKeown MJ (2021) Frequency-specific effects of galvanic vestibular stimulation on response-time performance in Parkinson’s disease. Front Neurol. https://doi.org/10.3389/fneur.2021.758122
Levin J, Kurz A, Arzberger T, Giese A, Hoglinger GU (2016) The differential diagnosis and treatment of atypical parkinsonism. Dtsch Arztebl Int 113:61–69
Liao K, Wagner J, Joshi A, Estrovich I, Walker MF, Strupp M, Leigh RJ (2008) Why do patients with PSP fall? Evidence for abnormal otolith responses. Neurology 70:802–809
McDonnell MD, Ward LM (2011) The benefits of noise in neural systems: bridging theory and experiment. Nat Rev Neurosci 12:415–426
Moss F, Ward LM, Sannita WG (2004) Stochastic resonance and sensory information processing: a tutorial and review of application. Clin Neurophysiol 115:267–281
Mulavara AP, Kofman IS, De Dios YE, Miller C, Peters BT, Goel R, Galvan-Garza R, Bloomberg JJ (2015) Using low levels of stochastic vestibular stimulation to improve locomotor stability. Front Syst Neurosci 9:117
Müller ML, Albin RL, Kotagal V, Koeppe RA, Scott PJ, Frey KA, Bohnen NI (2013) Thalamic cholinergic innervation and postural sensory integration function in Parkinson’s disease. Brain 136:3282–3289
Murdin L, Bronstein AM (2009) Head deviation in progressive supranuclear palsy: enhanced vestibulo-collic reflex or loss of resetting head movements? J Neurol 256:1143–1145
O’Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, Revesz T, Lees AJ (2008) Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 131:1362–1372
Pal S, Rosengren SM, Colebatch JG (2009) Stochastic galvanic vestibular stimulation produces a small reduction in sway in Parkinson’s disease. J Vestib Res 19:137–142
Pan W, Soma R, Kwak S, Yamamoto Y (2008) Improvement of motor functions by noisy vestibular stimulation in central neurodegenerative disorders. J Neurol 255:1657–1661
Respondek G, Stamelou M, Kurz C, Ferguson LW, Rajput A, Chiu WZ, van Swieten JC, Troakes C, Al Sarraj S, Gelpi E, Gaig C, Tolosa E, Oertel WH, Giese A, Roeber S, Arzberger T, Wagenpfeil S, Hoglinger GU, Movement Disorder Society-endorsed PSPSG (2014) The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord 29:1758–1766
Samoudi G, Jivegard M, Mulavara AP, Bergquist F (2015) Effects of stochastic vestibular galvanic stimulation and LDOPA on balance and motor symptoms in patients with Parkinson’s disease. Brain Stimul 8:474–480
Scelzo E, Lozano AM, Hamani C, Poon YY, Aldakheel A, Zadikoff C, Lang AE, Moro E (2017) Peduncolopontine nucleus stimulation in progressive supranuclear palsy: a randomised trial. J Neurol Neurosurg Psychiatry 88:613–616
Schniepp R, Boerner JC, Decker J, Jahn K, Brandt T, Wuehr M (2018) Noisy vestibular stimulation improves vestibulospinal function in patients with bilateral vestibulopathy. J Neurol 265:57–62
Seemungal B, Yousif N, Bronstein AM, Naushahi J, Nandi D (2010) POD06 Human pedunculopontine nucleus displays vestibular reactivity. J Neurol Neurosurg Psychiatry 81:e43
Serrador JM, Deegan BM, Geraghty MC, Wood SJ (2018) Enhancing vestibular function in the elderly with imperceptible electrical stimulation. Sci Rep 8:336
Slade SC, Finkelstein DI, McGinley JL, Morris ME (2020) Exercise and physical activity for people with progressive supranuclear palsy: a systematic review. Clin Rehabil 34:23–33
Slade SC, Underwood M, McGinley JL, Morris ME (2019) Exercise and progressive supranuclear palsy: the need for explicit exercise reporting. BMC Neurol 19:305
Stamelou M, Respondek G, Giagkou N, Whitwell JL, Kovacs GG, Hoglinger GU (2021) Evolving concepts in progressive supranuclear palsy and other 4-repeat tauopathies. Nat Rev Neurol 17:601–620
Stiles L, Smith PF (2015) The vestibular-basal ganglia connection: balancing motor control. Brain Res 1597:180–188
Tran S, Shafiee M, Jones CB, Garg S, Lee S, Pasman EP, Carpenter MG, McKeown MJ (2018) Subthreshold stochastic vestibular stimulation induces complex multi-planar effects during standing in Parkinson’s disease. Brain Stimul 11:1180–1182
Venhovens J, Meulstee J, Bloem BR, Verhagen WI (2016) Neurovestibular analysis and falls in Parkinson’s disease and atypical parkinsonism. Eur J Neurosci 43:1636–1646
Volter F, Beyer L, Eckenweber F, Scheifele M, Bui N, Patt M, Barthel H, Katzdobler S, Palleis C, Franzmeier N, Levin J, Perneczky R, Rauchmann BS, Sabri O, Hong J, Cumming P, Rominger A, Shi K, Bartenstein P, Brendel M (2023) Assessment of perfusion deficit with early phases of [(18)F]PI-2620 tau-PET versus [(18)F]flutemetamol-amyloid-PET recordings. Eur J Nucl Med Mol Imaging 50:1384–1394
Voros J, Rise R, Sherman S, Durell A, Anderson AP, Clark TK (2022) A machine learning approach to identify stochastic resonance in human perceptual thresholds. J Neurosci Methods 374:109559
Warren NM, Piggott MA, Perry EK, Burn DJ (2005) Cholinergic systems in progressive supranuclear palsy. Brain 128:239–249
Wright A, Hannon J, Hegedus EJ, Kavchak AE (2012) Clinimetrics corner: a closer look at the minimal clinically important difference (MCID). J Man Manip Ther 20:160–166
Wuehr M, Boerner JC, Pradhan C, Decker J, Jahn K, Brandt T, Schniepp R (2018) Stochastic resonance in the human vestibular system: noise-induced facilitation of vestibulospinal reflexes. Brain Stimul 11:261–263
Wuehr M, Decker J, Schniepp R (2017) Noisy galvanic vestibular stimulation: an emerging treatment option for bilateral vestibulopathy. J Neurol 264:81–86
Wuehr M, Eder J, Keywan A, Jahn K (2022) Noisy galvanic vestibular stimulation improves vestibular perception in bilateral vestibulopathy. J Neurol 270(2):938–943
Wuehr M, Nusser E, Decker J, Krafczyk S, Straube A, Brandt T, Jahn K, Schniepp R (2016) Noisy vestibular stimulation improves dynamic walking stability in bilateral vestibulopathy. Neurology 86:2196–2202
Wuehr M, Nusser E, Krafczyk S, Straube A, Brandt T, Jahn K, Schniepp R (2016) Noise-enhanced vestibular input improves dynamic walking stability in healthy subjects. Brain Stimul 9:109–116
Wuehr M, Schmidmeier F, Katzdobler S, Fietzek UM, Levin J, Zwergal A (2022) Effects of low-intensity vestibular noise stimulation on postural instability in patients with Parkinson’s disease. J Parkinsons Dis 12:1611–1618
Yamamoto Y, Struzik ZR, Soma R, Ohashi K, Kwak S (2005) Noisy vestibular stimulation improves autonomic and motor responsiveness in central neurodegenerative disorders. Ann Neurol 58:175–181
Zweig RM, Whitehouse PJ, Casanova MF, Walker LC, Jankel WR, Price DL (1987) Loss of pedunculopontine neurons in progressive supranuclear palsy. Ann Neurol 22:18–25
Zwergal A, la Fougere C, Lorenzl S, Rominger A, Xiong G, Deutschenbaur L, Linn J, Krafczyk S, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K (2011) Postural imbalance and falls in PSP correlate with functional pathology of the thalamus. Neurology 77:101–109
Acknowledgements
The authors would like to thank Lorenz Assländer for providing resources for data analysis. The study was supported by the German Federal Ministry for Education and Science (BMBF, 01EO1401 & 13GW0490B).
Funding
Open Access funding enabled and organized by Projekt DEAL. Bundesministerium für Bildung und Forschung, 01EO1401, Max Wuehr, 13GW0490B, Max Wuehr.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
MW received funding from the neuroConn GmbH (DC stimulator).
Ethical approval
The ethics committee of the medical faculty of the Ludwig-Maximilians-University approved the study protocol (20–1137), which was conducted in accordance with the Declaration of Helsinki.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wuehr, M., Peto, D., Fietzek, U.M. et al. Low-intensity vestibular noise stimulation improves postural symptoms in progressive supranuclear palsy. J Neurol 271, 4577–4586 (2024). https://doi.org/10.1007/s00415-024-12419-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-024-12419-9